Appendix E: Panelists’ Comments Submitted After the Meeting
Error processing SSI fileContents:
- Dr. Lippmann’s Post-Meeting Comments
- Dr. Lockey’s Post-Meeting Comments
- Dr. McConnell’s Post-Meeting Comments
- Dr. Mossman’s Post-Meeting Comments
- Dr. Oberdörster’s Post-Meeting Comments
- Dr. Wallace’s Post-Meeting Comments
Note:
The expert panelists were asked to provide premeeting comments and to participate in the discussions at the expert panel review meeting. In addition, several panelists chose to submit additional written comments after the expert panel review meeting. Some panelists submitted updated versions of their premeeting comments (see Appendix B), while others wrote summaries of the discussions they led at the expert panel review meeting. All post-meeting comments are presented here, regardless of their content. Panelists were not required to submit post-meeting comments.
This section presents the post-meeting comments exactly as they were submitted to ERG, with only minor changes to format and references. The expert panel was not asked to comment on the content of these post-meeting comments.
Note:
Dr. Case submitted post-meeting comments as a list of suggested revisions to an earlier draft of this report. Dr. Case’s comments have been incorporated directly into the text of this report and are not replicated here.
Dr. Lippmann’s Post-Meeting Comments
ATSDR Fiber Panel Review
Topic # 1. Physiological Fate of Asbestos and Vitreous Fibers less than 5 Microns in Length. Discuss/review current knowledge about the physiological fate of small fibers when they enter the body.
A. What is the expected physiological depositional pattern for less-than-5-micron fibers in the lung?
This is well established in terms of the depositional mechanisms of impaction, sedimentation, Brownian motion and (for fibers) interception. Fibers with aspect ratios >10 behave aerodynamically like unit density spheres with diameters three times their fiber width (Stöber et al., 1970; Timbrell, 1972). The only exception, in terms of being influential in deposition in lung airways is for fibers longer than about 10 µm, where the mechanism of interception becomes influential (Sussman et al, 1991). This also accounts for the fact that longer fibers have proportionately more deposition in the airways as opposed to peripheral alveoli. The fact that lung retention also increases more markedly with fibers greater than 10 microns is supported by theoretical calculations (Yu et al., 1990), analysis of lung dust content in humans (Timbrell, 1982; Churg and Wiggs, 1987; Pooley and Wagner, 1998) and studies using experimental animals (Morgan 1979, 1995). Thus, for fibers <5 µm in length, deposition patterns and efficiencies will be determined almost entirely according to the fiber width, which for fibers <5 µm long will be less than about 1.6 µm. For fiber widths between about 0.1 and 1.6 mm, total lung deposition in healthy people will be between 10 and 20%, with almost all of it in the deep lung. For fibers thinner than 0.1 µm, deposition will increase with decreasing width, and there will be a somewhat greater proportion of the deposition in the more proximal airways. Particles that are not deposited remain suspended in the tidal air and are exhaled.
There are significant differences between humans and rats with respect to deposition efficiencies of long as well as short fibers; respirability is very different and the deposition fractions are significantly different as well between the two species.
B. What is known about clearance/biopersistence of less-than-5-micron fibers once deposited in the lungs?
For these short fibers, which can be fully engulfed by lung cells and do not dissolve in airway fluids in less than a few weeks, their clearance will be similar to other mineral and vitreous particles. Those depositing in lung conductive airways will be largely removed to the G.I. tract by mucociliary clearance within about one day. Most of those depositing in the gas-exchange region will be phagocytized by alveolar macrophages and cleared to and through the mucociliary escalator within a few weeks. Other particles may be engulfed by epithelial cells, primarily in the vicinity of the bronchial-alveolar duct junctions, and retained for much longer periods, with gradual removal to lymph nodes.
The relatively rapid clearance of short fibers and compact particles from the lung has been demonstrated in a number of studies (reviewed in Health Effects Institute-Asbestos Research, 1991; Davis, 1994; Oberdörster et al., 1990; Morgan, 1995). Such particles can be: 1) readily transported through tracheobronchial and other lymph nodes to more distal lymphatics, the pleura, or other organs; 2) cleared via the mucociliary escalator and alveolar macrophages; and 3) effectively phagocytized by a number of cell types in the lung including epithelial cells (Churg et al., 2000). Once within a phagolysosome or in general in lung fluids, shorter fibers of chrysotile asbestos (Hume and Rimstidt, 1992) or glass (reviewed in Lippmann, 1990) are more prone to dissolution and fragmentation than longer fibers and amphibole types of asbestos.
Absent abnormalities in phagocyte function of these particles should be removed even if they are chemically resistant if: (a) the dose is not too great to overwhelm these normal mechanisms; and (b) the mechanisms themselves are intact. There are medical conditions which affect these mechanisms, however, so there are likely to be vulnerable populations (such as those with primary ciliary disorders; these tend to be genetic and very rare such as primary ciliary dyskinesia (incidence 1:20,000 to 1:60,000)). Of greater frequency is the lesser effect on mucociliary clearance in asthma. In addition environmental influences, including smoking and nitrogen dioxide (Case et al., 1982), can affect these normal mechanisms through direct ciliary damage or disrupted function. Some common pharmaceuticals slow mucociliary transport (for example, some general anaesthetics and atropine), while others accelerate it (for example, theophyllines and sympathomimetics). Bronchial secretion is also an important contributor to clearance or impaired clearance, as can be seen most dramatically in cystic fibrosis. Overall, then, there are a number of possible factors that may interfere with particle clearance, but none have been associated with “fiber length” parameters with the possible exception of smoking (Takahashi et al., 1994).
The most important physiological clearance mechanism in alveolar region is clearance by alveolar macrophages (AM). Of importance is fiber length with respect to phagocytosis and removal by alveolar macrophages. Short fibers are easily phagocytized, fibers longer than 20 µm are not. There are species differences in AM size. Thus, clearance for long fibers is prolonged, as is that for short fibers when high lung burdens are reached (particle overload). Also, intrinsic toxicity, which influences clearance, has to be considered. Inflammatory conditions in the lung (for example, smokers) also contribute to impairment of alveolar macrophage-mediated mechanical clearance and need to be considered.
Biopersistence is the sum of physiological clearance processes and physicochemical processes, which together account for the retention halftime of the fibrous or non-fibrous material in the lung. Physicochemical processes include dissolution, leaching, breaking and splitting, depending on the fibrous material, that can occur intra- as well as extra-cellularly, and differences in pH in both locations are of importance here. Clearance rates of fibers of different length categories have been determined from short- and long-term inhalation studies (Davis et al., 1986, 1987; Wagner, 1990). Generally, short fibers are cleared rapidly if biosoluble (pH differs intracellularly vs. extracellularly), or at rates similar to nonfibrous particles. Breakage of long fibers will give input into short fiber category.
The hazards associated with man-made vitreous fiber (MMVF) appear to be most strongly associated with the ability to persist within lung tissue. This is, in part, dependent upon chemical composition of the MMVF, in that increased concentrations of stabilizers such as aluminum impact a greater degree of chemical durability. In vitro tests to measure fiber solubility should be performed to reflect an acid pH of 4.5 to 5.0 such as found in phagolysomes within alveolar macrophages as well as pH of 7.4 reflecting extra-cellular fluid. Short fibers that are ingested by macrophages will encounter the lower pH that overall could affect their biopersistence. In general, solubility tests identified the following rank order from lowest to greatest solubility of MMVF in comparison to asbestos fibers: crocidolite <amosite <RCF <special purpose glass fibers <rock wool <slag wool <conventional glass fibers (NRC, 2000).
In rodent exposure to mixed dust resulted in an increased transport of fibers across the visceral pleura and increase production of lung tumors and mesothelioma (IARC# 140, 1996).
C. What type(s) of migration are expected within the body for less-than-5-micron fibers?
Fibers with diameters less than ~0.1 µm, which could be a significant fraction of fibers <5 µm in length, can penetrate through the respiratory epithelia and be transported through lymph channels to hilar and peripheral (mesothelial) lymph nodes and through blood to more distant body organs. Gelzleichter et al. (1996) exposed rats to nose only inhalation of kaolin-based refractory ceramic fiber. It was identified that fibers rapidly translocate to the pleural tissue with a difference between those in the pleural tissue and the parenchymal tissue. Within the pleural tissue the geometric mean length 1.5 µm (GSD ~ 2.0) and geometric mean diameter 0.09 µm (GSD ~1.5). For comparison parenchymal tissue GML = 5.0 µm (GSD ~2.3) and GMD 0.3 µm (GSD ~1.9.) This would indicate the short thin fibers are capable of translocating to the pleural tissue.
This may be an important subject, at least for the parietal pleura, if it is necessary for fibers to reach the pleura to cause lesions (plaques and mesothelioma). It remains possible that fibers still within the peripheral lung may be capable of contributing to the mechanisms of these diseases. Mechanisms remain speculative, but long amphibole fibers may tend to localize toward the lung periphery, and it remains possible (but unproven and indeed untested) that chemical mediators may cross the visceral pleura into the pleural space. Churg and Wiggs (1987), among others, have observed that “accumulation of long fibers immediately under the upper lobe pleura may be important in the genesis of mesothelioma.”
Two recent studies are informative (Boutin et al., 1996; Dumortier et al., 2002). They found that “the distribution of asbestos fibers in the pleura was heterogeneous and that they might concentrate in…’black spots’ of the parietal pleura.” Using thoracoscopy in living patients from “normal areas of the parietal pleura” rather than plaques and tumor, and using controls, they showed that “amphiboles outnumbered chrysotile in all samples” and that of all fibers 22.5% were in fact greater than or equal to 5 µm in length; a proportion at least as great as that usually seen in lung tissue. The means of translocation remains unknown, although these findings strongly suggest lymphatic drainage paths. The pathogenic significance also remains unknown, although the authors emphasized their hypothesis that these fibers might contribute to plaque and mesothelioma genesis.
Other papers that have been published (in relation to human disease) have been for the most part based on static “fiber burdens” that purport to be in “the pleura” but which on careful reading are in fact in mesotheliomatous tissues and/or pleural plaques; the false assumptions are then made that “short fibers” - usually very short chrysotile fibers, averaging less than 0.2 µm in length - have “translocated” to the “pleura” from the lung. In fact the “pleura” was not studied, tumor and plaque, which by definition could not contain fibers except via specimen contamination or incorporation, most likely from adjacent lung. Both Rogers et al. (1994) and Case et al. (1994) have also reported contamination by short crocidolite fibers of Nuclepore filter materials and in uncontrolled studies of this nature any material from air, fluids, and paraffin in the pathology laboratory from which the specimens originally were referred to specimen preparation materials are suspect.
References
Boutin, C., P. Dumortier, F. Rey, J.R. Viallat, and P. De Vuyst. 1996. Black spots concentrate oncogenic asbestos fibers in the parietal pleura. Thoracoscopic and mineralogic study. Am. J. Respir. Crit. Care Med. 153:444-449.
Case, B.W., R.E. Gordon, and J. Kleinerman. 1982. Acute bronchiolar injury following nitrogen dioxide exposure: A freeze fracture study. Environ. Res. 29:399-413.
Case, B.W.K., M. Harrigan, and A. Dufresne. 1994. Lung fibre content of American children aged 8-15 years. Ann. Occup. Hyg. 38:639-645.
Churg, A. and B. Wiggs. 1987. Accumulation of long asbestos fibers in the peripheral upper lobe in cases of malignant mesothelioma. Am. J. Ind. Med. 11:563-569.
Churg, A., J. Wright, B. Gilks, and J Dai. 2000. Pathogenesis of fibrosis produced by asbestos and man-made mineral fibers: What makes a fiber fibrogenic? Inhal. Toxicol. 12:15-26.
Davis, J.M.G. 1987. Experimental data relating to the importance of fibre type, size, deposition, dissolution and migration. In: Proceedings of 1987 Mineral Fiber Symposium. Lyons: International Agency for Research on Cancer.
Davis, J.M.G. 1994. The role of clearance and dissolution in determining the durability of biopersistence of mineral fibers. Environ. Health Perspect. 102:113-117.
Davis, J.M.G., J. Addison, R.E. Bolton, K. Donaldson, A.D. Jones, and T. Smith. 1986. The pathogenicity of long versus short fiber samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br. J. Exp. Pathol. 67:415-430.
Dumortier, P., F. Rey, J.R. Viallat, I. Broucke, C. Boutin, P. De Vuyst. 2002. Chrysotile and tremolite asbestos fibres in the lungs and parietal pleura of Corsican goats. Occup. Environ. Med. 59:643-646.
Gelzleichter T.R., E. Bermudez, J.B. Mangum, B.A. Wong, J.I. Everitt, and O.R. Moss. 1996. Pulmonary and pleural responses in Fischer 344 rats following short-term inhalation of a synthetic vitreous fiber. I. Quantitation of lung and pleural fiber burdens. II. Pathobiologic responses. Fundam. Appl. Toxicol. 30:31-46.
HEI-AR Asbestos Literature Review Panel. 1991. Asbestos in public and commercial buildings: A literature review and synthesis of current knowledge. Cambridge, MA: Health Effects Institute - Asbestos Research.
Hume, L.A. and J.D. Rimstidt. 1992. The biodurability of chrysotile asbestos. Am. Mineral 77:1125-1128.
IARC. 1996. Mechanisms of Fibre Carcinogenesis. In: IARC Scientific Publications No. 140, eds. A.B. Kane, P. Bofetta, R. Saracci, and J.D. Wilbourn. Lyon: International Agency for Research on Cancer.
Lippmann, M. 1990. Effects of fiber characteristics on lung deposition, retention, and disease. Environ. Health Perspect. 88:311-317.
Morgan, A. 1995. Deposition of inhaled asbestos and man-made mineral fibres in the respiratory tract. Ann. Occup. Hyg. 39:747-758.
NRC. 2000. Review of the U.S. Navy's Exposure Standard for Manufactured Vitreous Fibers. Washington, DC: National Academy Press.
Oberdörster, G., J. Ferin, J. Finkelstein, S. Soderholm, and R. Gelein. 1990. Mechanistic studies on particle-induced acute and chronic lung injury. In: Aerosols: Science, Industry, Health and Environment, Vol. 2, eds. S. Masuda and K. Takahashi, pp. 1229-1233. New York, NY: Pergamon Press.
Rogers A.L, J. Berry, et al. 1994. Dose-response relationship between airborne and lung asbestos fibre type, length, and concentration, and the relative risk of mesothelioma. Ann. Occup. Hyg. 38:631-638.
Stöber, W., H. Flachsbart, and D. Hochrainer. 1970. Der aerodynamische Durchmesser von Latexaggregaten and Asbestfasern. Staub-Reinhalt. Luft. 30:277-285.
Sussman, R.G., B.S. Cohen, and M. Lippmann 1991a. Asbestos fiber deposition in a human tracheobronchial cast. I. Experimental. Inhal. Toxicol. 3:145-160.
Sussman, R.G., B.S. Cohen, and M. Lippmann 1991b. Asbestos fiber deposition in a human tracheobronchial cast. II. Empirical model. Inhal. Toxicol. 3:161-179.
Takahashi, K., B.W. Case, A. Dufresne, R. Fraser, T. Higashi, and J. Siemiatycki. 1994. Relation between lung asbestos fibre burden and exposure indices based on job history. Occup. Environ. Med. 51:461-469.
Timbrell, V. 1972. An aerosol spectrometer and its applications. In: Assessment of Airborne Particles, eds. T.T. Mercer, P.E. Morrow, and W. Stöber, pp. 290-330. Springfield, IL: Charles C. Thomas.
Timbrell, V. 1982. Deposition and retention of fibres in the human lung. Ann. Occup. Hyg. 26:347-369.
Wagner, J.C. 1990. Biological effects of short fibers. In: Proceedings of the VII International Pneumoconiosis Conference (Pittsburgh, PA, August 1988). NIOSH 90-108, Vol. 2, pp. 835-840. Washington, DC: National Institute of Occupational Safety and Health.
Dr. Lockey’s Post-Meeting Comments
What do human/epidemiological data tell us about small fibers? Discussion Leaders: Dr. Lockey and Dr. Case
Cancer Effects
Short natural occurring fibers. A study by Higgins, et al. [1] in 1983 reviewed the mortality of workers employed at the Reserve Mining Company at Babbit, Minnesota. These workers were involved with mining taconite, which is a dense hard rock composed of silica, silicates and iron. Taconite mined in the eastern tip of the Mesabi range contained amphiboles in the cummingtonite-grunerite series. These fibers are short in length with reportedly the vast majority being <10 µm and are related to amosite asbestos. Of the 9,065 men employed by the company as of July 1, 1976, 5,751 had worked one year or more. The investigators established the vital status of 96% of those who worked for five years or longer and 75% of former workers who worked one to four years. The total respirable dust ranged from 0.02 mg/m3 to 2.52 mg/m3 and as high as 2.75 mg/m3 with the modal range from 0.2 mg/m3 to 0.6 mg/m3. There were relatively few measurements of fibers and those that were available demonstrated concentrations usually low with a few at or above 0.5 fibers/ml in the crushing department. Reportedly none approached the OSHA threshold limit value which at that time was 2 fibers/ml. Results of the study indicated that there was no excess death in this population including those men with cumulative exposure of 1,000 to 3,000 total dust years or 500 to 1,000 silica dust years. The conclusions of the study indicated the death rates for all causes were significantly below expectations including selected respiratory disease and death from malignant disease was marginally below that expected for the State of Minnesota. There was no relationship between lifetime dust exposure and increased mortality, nor was there any indication that malignant neoplasm was increased after 15 to 20 years latency. The authors identified a weakness of the study in that the average latency of the cohort was 14.7 years with a maximum of 24.6 years, or a relatively short latency for development of cancer.
A study by McDonald, et al. [2] regarding the mortality from long-term exposure to cummingtonite-grunerite from a gold extraction process at the Homestake Mine, Lead, South Dakota was reviewed. Those workers who had worked 21 years or longer were traced and of 660 men who had died, the cause of death was ascertained for 657. Results of the study indicated pneumoconiosis, which was mainly silicosis along with tuberculosis, and heart disease were causes of excess death. There was a dust exposure relationship for both pneumoconiosis and respiratory tuberculosis, but reportedly no convincing increase in respiratory cancer. It was noted that more than 75% of the 660 men who had died started to work before 1925. The interval between first employment and death and the 76 fatalities from tuberculosis or pneumoconiosis ranged from 22 to 61 years with a median of 35 years. Average silica dust concentrations ranged from 11.0 to 24.6 mppcf before 1952. A study by Dement, et al. [3] reported that 80% to 90% of fibers in the mine had an amphibole diffraction pattern by transmission/scanning electron microscope equipped with an energy-dispersive X-ray spectrometer. The mean total fiber concentration was 4.82 ± 0.68 f/cc (range 0.66–11.79) with 0.36 ± 0.08 f/cc (range 0.07-1.29) greater than 5 µm in length. There was one potential mediastinal mesothelioma which could not be confirmed in the 17 respiratory malignancies (16.5 expected based on South Dakota rates). The results of the study were in conflict with an earlier study by Gillam, et al of the same mine of 440 males who worked at least five years underground by 1960. Reportedly there were 10 deaths from neoplasm of the respiratory system between 1960 and 1973 where as 2.7 were expected based on the male population of South Dakota [4].
Conclusions
- There is no data regarding human exposure to asbestos fiber uniformity less
than 5 µm in length.
- Studies of workers exposed to cummingtonite-grunerite, a type of amphibole
related to amosite, demonstrated no consistent increase in overall mortality,
mortality related to selected respiratory disease, or respiratory cancer.
The vast majority of airborne fibers were reported to be less than 10 µm
in length.
- Studies of workers of a gold mine in Lead, South Dakota exposed to cummingtonite-grunerite
initially demonstrated an increased mortality from malignant respiratory disease.
A subsequent study did not confirm the initial finding but demonstrated an
increase in silicosis and tuberculosis. Mean total fiber concentration was
4.82 f/cc with 0.36 f/cc greater than 5 µm in length.
- Consideration should be given for performing a feasibility study regarding an updated mortality analysis of these two cohorts.
MMVF Mortality Studies. Mortality studies of glass fiber and mineral wool production workers have been ongoing in the U.S. most recently under the direction of Marsh, et al at the University of Pittsburgh, and within the European Union under the direction of the International Agency for Research on Cancer (IARC). The most recent follow up study by Marsh, et al. [5,6,7] of 10 U.S. glass fiber manufacturing plants demonstrate no excess mortality from all causes, all cancers combined, or non-malignant respiratory disease. For respiratory system cancer, there was an observed 6% excess that was statistically significant for the total cohort but not found in workers who had five or more years of employment. An association was seen with calendar time and time since first employment, but no relationship was found with duration of employment, or increase in exposure to respirable glass fiber. A case-control study of respiratory system cancer did not identify increased risk with duration of exposure, cumulative exposure, or time since first employment. An association with non-baseline levels of average intensity of exposure to respiratory fibers was not present when adjusted for smoking.
A previous case-control study of a glass fiber manufacturing facility included in the U.S. glass fiber study demonstrated that differences in local versus national smoking rates may have been a contributing factor in the excess respiratory cancer seen in that manufacturing facility. [8] The potential confounding impact of cigarette smoking in the U.S. glass fiber and rock/slag wool studies was further explored by Buchanich, et al. [9] and Marsh, et al. [10] and identified as the potential unaccounted for factor regarding the small excess respiratory system cancer not related to exposure indices.
Previous analysis of five rock and slag wool plants in the U.S. demonstrated increased lung cancer mortality using U.S. but not local rates, and this was confined to short-term workers or those workers with less than five years duration of employment. There was no association with measures of respirable fiber exposure. [11] Within the U.S. a case-control study of 9 slag wool plants demonstrated an association with smoking but not MMVF exposure. [12]
Most recent analysis of the U.S. rock and slag wool workers as well as glass fiber production workers identified ten death certificates that mentioned the term mesothelioma. [13] Of the ten cases of mesothelioma, two on pathology review were definitely not felt to be mesotheliomas, one had a 50% chance of mesothelioma, and two others had less than 50% chance of mesothelioma. Medical records or pathology specimens were not available on the remaining five. Using a timeframe when specific malignant mesothelioma coding rubrics were available, the expected mesothelioma rate (local county comparison) was 2.19 versus 1 observed. Overall the authors felt there was no increased risk from the malignant mesothelioma in the U.S. MMVF cohort.
The IARC have followed the mortality of workers among 13 MMVF manufacturing facilities in Europe. [14] The most recent update demonstrated a significant increase in lung cancer mortality in rock and slag wool workers as well as glass wool workers, using national mortality rates which disappeared for the glass wool workers when using local adjustment factors to the national mortality rates. In addition, there was no association in the glass wool workers with time since initial employment or duration of employment, and with removal of glass wool workers with less than one-year employment no excess lung cancer was noted.
Within the rock and slag wool cohort there was an increase in lung cancer risk
but the authors felt there was no clear information to indicate that the increased
cancer risk was specifically related to fiber exposure. [14] A subsequent cohort
study demonstrated similar results. [15] A case-control study nested in this
latter cohort indicated no relationship between cumulative rock or slag wool
exposure and lung cancer. [16,17]
Within the IARC study there were five cases of mesothelioma, two which occurred
in workers with less than one-year employment and two in workers with most likely
prior asbestos exposure. [14]
Preliminary results of a mortality study of U.S. RCF manufacturing workers demonstrate no significant increase in malignant or non-malignant respiratory mortality and no malignant mesothelioma. The power of the study was limited as the cohort was relatively young and small in number. [18]
Conclusions
- There are no data regarding human exposure to MMVF uniformity less than
5 µm in length.
- There is no persuasive evidence that exposure to glass fiber, rock wool,
slag wool, or refractory ceramic fiber has been associated with increased
lung cancer risks based on ongoing U.S. and European mortality studies.
- There is no indication of an increased risk for mesothelioma.
Non-Cancer Effects
MMVF Morbidity and Mortality Studies. Non-malignant respiratory effects: Studies of five fiberglass and two mineral wool manufacturing facilities identified small opacities in 1.6 % of the population studied that were predominantly irregular in shape. [19] These workers were involved with working in facility manufacturing fibers over 3 µm in diameter and fibers averaging 1 µm to 3 µm in diameter. The overall rate of chest X-ray changes was no different in comparison to a non-MMVF exposed comparison group, and any relationship between exposure indices was seen at profusion level 1/0 but not 1/1. There was no increase in upper or lower respiratory tract symptoms. Similar results were seen in a study in Australia of glass and rock wool production workers with no findings of asthma, pulmonary fibrosis or pleural disease. [20] A similar study of rock wool workers also did not demonstrate increased respiratory symptoms or abnormalities with DLCO or DL/Va. A potential additive or synergistic effect, however, was seen regarding the FEV1/FVC ratio, fiber exposure, and those with greater than 40-pack year history of cigarette smoking. [21]
The IARC [22] study demonstrated no increased mortality from asthma, bronchitis or emphysema, which is similar to the most recent analysis of the glass fiber workers in the United States which did not identify increased mortality from non-malignant respiratory disease. [5] Of interest in the IARC study was the suggestion of an increased risk from non-malignant renal disease (SMR 0.97, 95% CI 0.36 to 2.11) in regard to duration of employment or employment at an early phase within the rock and slag wool industry. Within the U.S. mineral wool study a similar trend was noted (p < .05) with a SMR of 204 (observed 12) in regard to nephritis and nephrosis. [11] Similar type patterns have not been demonstrated in relationship to nephritis and nephrosis deaths in U.S. glass wool manufacturing facilities. [23]
There are very limited studies on end users of man-made vitreous fibers. One study identified increased prevalence of chest radiograph evidence of irregular opacities in workers using rotary spun fiberglass, but there was a question of airborne asbestos fibers within the plant site. [24,25] In insulators a decrease in FEV1 was identified in comparison to a non-exposed control group after adjusting for smoking habits and self-assessed former asbestos exposure. [26]
On-going morbidity studies of workers involved with refractory ceramic fiber (RCF) manufacturing have identified a relationship between pleural plaques and time from initial employment, duration of employment, and cumulative refractory ceramic fiber exposure. Pleural changes were seen 2.7% or 27 workers out of 1,008 of which 22 were pleural plaques. Of those with greater than 20 years latency from initial production job or 20 years duration in a production job, 16 workers or 8.0% and 5 workers or 8.1% had pleural changes, respectively. Interstitial changes were noted in 1.0% at profusion category >1/0, similar to other non-specified dust exposed worker populations and showed a non-significant elevated OR in regard to cumulative fiber exposure of 4.7 (95% CI, 0.97 to 23.5). In regard to cumulative fiber exposure, 5.4% (8 of 148) with greater than 45 to 135 fiber-month/cc exposure had pleural changes (OR 5.6, 95% CI, 1.5 – 28.1). For those with >135 fiber-months/cc exposure, 9.8% (6 of 61) had pleural changes (OR 6.0, CI 1.4 – 31.0). [27] European studies concurred that there was some evidence of a relationship between RCF latency and pleural changes including pleural plaques but not duration or intensity of RCF exposure, but it was difficult to separate the effects asbestos and RCF exposure and any relationship between RCF exposure and small opacities was at best ambiguous. [28]
Previous studies of the RCF workers demonstrated a relationship between 10 years of employment in production job tasks prior to 1987 and small decrements in FVC for current (165.4 ml) and past (155.5 ml) male smokers, but not never-smokers, and small decrements in FEV1 for current male smokers only (134.9 ml). For never-smoker women there was also a decrement in FVC (350.3 ml) per 10-years employment in production job tasks. [29] A longitudinal analysis in those male workers able to provide five tests or more did not demonstrate any further decrement of the FEV1 or FVC between initial and final tests. [30]
Conclusions Regading Non-Cancer Effects of MMVF
- There are no available morbidity studies of workers exposed to MMVF uniformly
less than 5 µm in length.
- No increased mortality from non-malignant respiratory disease.
- No indication of chest radiograph interstitial or pleural changes in regard
to glass and mineral wool production workers but data is limited.
- Refractory ceramic fiber (RCF) exposure appears to be associated with the
occurrence of pleural plaques that most likely are related to increased exposure
levels in the RCF manufacturing facilities prior to 1985.
- Potential additive or synergistic effect with MMVF exposure and small decrement
in FVC and/or FEV1 involving current or former smokers.
- Within mineral wool cohort, question of potential increased mortality from
non-malignant renal disease such as nephritis and nephrosis.
- End user studies of MMVF users are limited and are confounded by potential previous asbestos exposure.
Irritant Effects
MMVF can cause skin irritation particularly in an area where clothing comes in close contact to the skin such as around the neck or forearms. Essentially this occurs in 5% of new workers involved with MMVF production. [31] Residential contamination of man-made vitreous fibers in high concentration can also cause irritation to the upper as well as lower respiratory tract. [32] Glass fibers with diameters greater than 5.3 µm have been reported to be more likely to cause skin irritation than the smaller diameter fibers, mainly due to mechanical irritation. [33,34] There has been documentation of eye irritation associated with MMVF as well as nasal and pharyngeal irritation with unusual MMVF dust exposure situations. [35,36]
Conclusions
- Skin irritation appears to be related to the mechanical effects of fiber ~5 µm in diameter and appears to be worse in hot, humid weather.
- Accidental exposure to increased concentrations of MMVF can result in upper and lower respiratory tract irritation as well as eye irritation.
Association Between Fiber Length and Fiber-like Toxicity
There have been no published studies that address whether asbestos fibers uniformly <5 µm in length have been associated with pleural or parenchymal disease in human. Any potential risk associated with fiber exposure <5 µm in length most likely would be related to an increased risk for pulmonary asbestosis, and most likely would occur at a substantially higher dose in comparison to exposures to asbestiform fibers (long fibers with high aspect ratios). [37]
There is some indication that fibers with diameters with <0.1 µm to 0.4 µm and lengths <10 µm may have a propensity for inducing pleural plaques. [38] Methodologies used to analyze pleural and/or parenchymal tissue for the presence of fibers and association of pleural changes differ markedly between investigators, however. Human studies of individual exposed to asbestos fibers are difficult to interpret in regard to toxicity solely related to fibers <5 µm in length because exposure situations almost uniformly contain a broad distribution of fiber diameters and length.
Preliminary results of residents of Libby, Montana that were exposed to asbestiform tremolite indicate a high propensity for pleural changes in comparison to interstitial changes. [39] There is some indication that exposure to tremolite fibers with relatively low aspect ratios in comparison to the asbestiform type tremolite may be capable of causing pleural plaques. [40] Pleural plaques can occur with minimal exposure to asbestos and can occur within a wide range of tissue burdens of asbestos fibers which overlap with control populations. [41]
Conclusions
- Even though there are no human studies solely of MMVF <5 µm in
length, the available morbidity and mortality studies of MMVF production workers
indicate limited overall toxicity from MMVF exposure.
- There are no human studies regarding exposures solely to asbestos fiber
<5 µm in length but there has been some speculation that durable
fibers <10 µm in length and <0.1 to 0.4 µm in diameter may
be associated with pleural plaques in relatively low concentration, in particular
the amphibole tremolite.
- For asbestos fibers <5 µm in length, it would appear that very high doses may have the propensity to cause interstitial fibrosis, particularly if the fibers are durable within intracellular fluids.
Thresholds for Toxic Action
For asbestos and MMVF less than 5 µm in length, thresholds for toxic action in humans have not been established but most likely is substantially higher than the thresholds for long durable fibers with increased aspect ratios of respirable size.
Asbestos Versus MMVF
Based on animal and human studies, natural occurring asbestos fibers that are of respiratory size, long and thin with high aspect ratios, and durable within physiologic fluids represent the highest risk for malignant (lung cancer and mesothelioma) and non-malignant (interstitial fibers) respiratory disease. These abnormalities have not been demonstrated in MMVF manufacturing workers.
References
1) Higgins ITT, Glassman JH, Oh MS, Cornell RG. Mortality of reserve mining company employees in relation to taconite dust exposure. Am J Epidemiol 1983;118(5);710-719.
2) McDonald JC, Gibbs GW, Liddell FDK, McDonald AD. Mortality after long exposure to cummingtonite-grunerite. Am Rev Respir Dis 1978;118:271-277.
3) Gillam JD, Dement JD, Lemen JM, Wagoner JK, Archer VE, Blejer HP. Mortality patterns among hard rock gold miners exposed to an asbestiform mineral. NY Acad Sci 1976:271-336.
4) Dement JM, Zumwalde RD, Wallingford KM. Discussion paper: Asbestos fiber exposure in a hard rock gold mine. Ann NY Acad Sci. 1976:271-345.
5) Marsh GM, Youk AO, Stone RA, Buchanich JM, Gula MJ, Smith TJ, Quinn MM. Historical cohort study of US man-made vitreous fiber production workers: I. 1992 fiberglass cohort follow-up: Initial finding. JOEM 2001;43(9):741-756.
6) Youk, AO, Marsh GM, Stone RA, Buchanich JM, Smith TJ. Historical cohort study of U.S. man-made vitreous fiber production workers: III. Analysis of exposure-weighted measures of respirable fibers and formaldehyde in the nested case-control study of respiratory system cancer. JOEM 2001;43(9):767-778.
7) Stone RA, Youk AO, Marsh GM, Buchanich JM, McHenry MB, Smith TJ. Historical cohort study of U.S. man-made quantitative exposure-response analysis of the nested case-control study of respiratory system cancer. JOEM 2001;43(9):779-792.
8) Chiazze L, Watkins DK, Fryan C. A case-control study of malignant and non-malignant respiratory disease among employees of a fiberglass manufacturing facility. Br J Ind Med 1992;49:326-331.
9) Buchanich JM, Marsh GM, Youk AO. Historical cohort of U.S. man-made vitreous fiber production workers: V. Tobacco-smoking habits. JOEM 2001;43(9):793-802.
10) Marsh GM, Buchanich JM, Youk AO. Historical cohort study of U.S. man-made vitreous fiber production workers: VI. Respiratory system cancer standardized mortality ratios adjusted for the confounding effects of cigarette smoking. JOEM 2001;43(9):803-808.
11) Marsh G, Stone R, Youk A, et al. Mortality among United States rock wool and slag wool workers: 1989 update. J Occup Health Safety-Anst NZ 1996;12:297-312.
12) Wong O, Foliart D, Trent LS. A case-control study of lung cancer in a cohort of workers potentially exposed to slag wool fibres. Br J Ind Med 1991;48:818-824.
13) Marsh GM, Gula MJ, Youk AO, Buchanich JM, Churg A, Colby TV. Historical cohort study of U.S. man-made vitreous fiber production workers: II. Mortality from mesothelioma. JOEM 2001;43(9):757-766.
14) Boffetta P, Saracci R, Andersen A, et al. Cancer mortality among man-made vitreous fiber production workers. Epidemiology 1997;8:259-268.
15) Boffetta P, Anderson A, Hansen J, et al. Cancer incidence among European man-made vitreous fiber production workers. Scan J Work Environ Health1999;25:222-226.
16) Kjaerheim K, Boffetta P, Hansen J, Cherrie J, Chang-Claude J, Eilber U, Ferro G, Guldner K, Olsen JH, Plato N, Proud L, Saracci R, Westerholm P, Andersen A. Lung cancer among rock and slag wool production workers. Epidemiology 2002 Jul;13(4):445-453.
17) IARC monographs on the evaluation of carcinogenic risks to humans. Volume 81, Man-made Vitreous Fibres 2002.
18) Lemasters G, Lockey J, Levin L, Yiin J, Reutman S, Papes D, Rice C. A longitudinal study of chest radiographic changes and mortality of workers in the refractory ceramic fiber industry. 2001 Congress of Epidemiology Abstracts Am J Epidemiology 2001;153(11):S264.
19) Hughes JM, Jones RN, Glindmeyer HW, et al. Follow up study of workers exposed to man made mineral fibres. Br J Ind Med 1993;50:658-666.
20) Woodcock AJ, Mellis CM. Respiratory health of workers in the Australian glasswool and rockwool manufacturing industry. Final report prepared for Insulation Wools Research Advisory Board by Institute of Respiratory Medicine. Royal Prince Alfred Hospitals. Sydney, Australia. 1994.
21) Hansen EF, Rasmussen FV. Hardt F, Kamstrup O. Lung function and respiratory health of long-term fiber-exposed stonewool factory workers. Am J Respir Crit Care Med 1999;160:466-472.
22) Sali D, Boffetta P, Anderson A, et al. Non-neoplastic mortality of European workers who produce man made vitreous fibres. Occup Environ Med 1999;56:612-617.
23) Chiazze L, Watkins DK, Fryan C. Fayerweather W, Bender JR, Chiazze M. Mortality from nephritis and nephrosis in the fiberglass manufacturing industry. Occup Environ Med 1999;56:164-166.
24) Kilburn KH, Powers D, Warshaw RH. Pulmonary effects of exposure to fine fiberglass: Irregular opacities and small airways obstruction. Br J Ind Med 1992;49:714-720.
25) Bender JR. Pulmonary effects of exposure to fine fiberglass: Irregular opacities and small airways obstruction. Br J Ind Med 1993;50:381-382.
26) Clausen J, Netterstrom B, Wolff C. Lung function in insulation workers. Br J Ind Med 1993;50:252-256.
27) Lockey JE, LeMasters GK, Levin L, Rice C, Yiin J, Reutman S, Papes D. A longitudinal study of chest radiographic changes of workers in the refractory ceramic fiber industry. Chest 2002;121:2044-2051.
28) Cowie HA, Wild P, Beck J, Auburtin G, Piekarski C, Massin N, Cherrie JW, Hurley JF, Miller BG, Groat S, Soutar CA. An epidemiological study of the respiratory health of workers in the European refractory ceramic fibre industry. Occup Environ Med 2001;58:800-810.
29) Lemasters G, Lockey J, Rice C, et al. Radiographic changes among workers manufacturing refractory ceramic fibre and products. Ann Occup Hyg 1994;38(1):745-751.
30) Lockey JE, Levin LS, Lemasters GK, et al. Longitudinal estimates of pulmonary function in refractory ceramic fiber manufacturing workers. Am J Respir Crit Care Med 1998;157:1226-1233.
31) Bjornberg A. Glass fiber dermatitis. Am J Ind Med 1985;8:395-400.
32) Newhall HH, Brahim SA. Respiratory response to domestic fibrous glass exposure. Environ Res 1976;12:201-207.
33) Possick PA, Gellin GA, Key MM. Fibrous glass dermatitis. Am Ind Hyg Assoc J 1970;31:12-15.
34) Heisel EB, Mitchell JH. Cutaneous reaction to fiberglass. Ind Med Surg 1957;26:547-550.
35) Stokholm J, Norn M, Schneider T. Ophthalmologic effects of man made mineral fibers. Scand J Work Environ Health 1982;8:185-190.
36) Milby TH, Wolf CR. Respiratory tract irritation from fibrous glass inhalation. J Occup Med 1969;11:409-410.
37) Lippmann M. Asbestos exposure indices. Environ Res 46:86-108,
1988.
38) Lentz TJ, Rice CH, Lockey JE, Succop PA, Lemasters GK. Potential significance
of airborne fiber dimensions measured in the U.S. refractory ceramic fiber manufacturing
industry. Am J Ind Med 1999;36:286-298.
39) Year 2000 Medical testing of individuals potentially exposed to asbestoform minerals associated with vermiculite in Libby, Montana. A report to the community. August 23, 2001. ATSDR.
40) American Thoracic Society. Medical Section of the American Lung Association. Health effects of tremolite. Am Rev Respir Dis 1990;142:1453-1458.
41) Hillerdal G. Pleural plaques: incidence and epidemiology, exposed workers and the general population; a review. Indoor Built Environ 1997;6:86-95.
Dr. McConnell’s Post-Meeting Comments
How Do Animal/Experimental Data Augment Our Understanding of Human Health Effects
Background: There have been numerous studies of the effects of various types of asbestos (ATSDR, in press) and SVFs (ATSDR, in press) in animals. Both fibrous and nonfibrous particulates have been used. Most studies have been conducted in rats and hamsters, but others, including nonhuman primates have been used. Routes of exposure have included inhalation (whole-body and nose-only), intratracheal instillation, intrapleural implantation/injection, intraperitoneal injection and ingestion. All of the routes of administration have their strengths and weaknesses (advantages, disadvantages and limitations) for use for assessing potential health effects in humans (McConnell, 1995). However, the inhalation route appears to produce the most relevant data because it is the only route that duplicates all aspects of human fiber exposure and disease (inflammation, fibrosis, lung cancer and mesothelioma) resulting from the exposure (McClellan et al., 1992). Also, the neoplastic changes typically occur late in the rodents’ life, similar to what occurs in humans exposed to asbestos. Other routes of exposure are also useful for comparing the toxic potential of various types of fibers and understanding the mode of action and many of the mechanisms of fiber toxicity and carcinogenicity. Additionally, the oral route (ingestion) appears to be the most appropriate route of exposure for studying the potential hazard of ingested asbestos.
Cancer effects: Rats and hamsters are the most frequently used species for assessing the potential carcinogenic effects as asbestos (IARC, 1987) and SVFs (IARC, 2002) and have been used with various routes of exposure. Of the two species, the rat appears to be the most appropriate one because it exhibits both lung cancer and mesothelioma in response to inhalation of known human carcinogenic fibers, e.g. asbestos. The hamster can be a useful model if one is only interested in the inflammatory, fibrogenic and mesotheliogenic effects of particulates. However, the hamster does not develop lung cancer after exposure to high levels of either chrysotile (McConnell et al., 1995) or amosite asbestos (McConnell et al., 1999). Other species have been used but have significant limitations that preclude their general use for carcinogenic bioassays. For example, the mouse is not as useful as the rat or hamster because its terminal airways are smaller and therefore, particulates of a mean mass aerodynamic diameter (MMAD) of greater than >0.5 um cannot reach the deep lung (alveolar region) which is the site of primary disease. Non-human primates would be an ideal animal model but are precluded because of their long life-span (would require at least 20-30 years to demonstrate a noncarcinogenic effect), availability (a cancer bioassay requires >200 animals/sex), and expense (such a study would cost >$20 million.
Most chronic rodent inhalation bioassays of asbestos have been conducted in rats, have not shown significant strain differences and males and females are equally sensitive to its carcinogenic effects (ATSDR, in press). The only large series of studies of various types of asbestos showed that if there is a gender difference, males might be slightly more responsive (Wagner, et al., 1974). Therefore, either sex is appropriate with males slightly more preferable. Just as importantly, both sexes are probably not necessary. However, these same studies have shown that while life-time exposure to asbestos may not be necessary, it is important to observe the animals for most of their life-span (see below).
The types of cancer induced by asbestos and SVFs in rodents are comparable to those observed in humans, although the preponderance of a given type and its biologic behavior appears to be species specific. In inhalation studies in rats the preponderant form of lung cancer is bronchoalveolar in origin, arising from type II alveolar cells. They occur late in the animal’s life, usually after 21 months of age. This is why lifetime studies may be necessary to fully exonerate a fiber from being considered carcinogenic. The tumors are slow growing and only occasionally are the cause of death. The biological sequence of growth is typically from bronchoalveolar hyperplasia to bronchoalveolar adenoma to bronchoalveolar carcinoma, although all aspects of the sequence of progression may not be found in a given lesion (Boorman and Eustis, 1990). Squamous cell metaplasia is not unusual and typically is found as part of the morphology of larger tumors. Squamous cell carcinoma may predominate in a small percentage of rodent tumors, but has rarely been observed to occur de novo. Squamous cell types may be more common with intratracheal instillation of the fibers (Pott et al., 1994). The malignant tumors are locally invasive and can metastasize but it is an unusual event for them to do so. When this occurs it is usually within the lung, but distant metastases have been observed. The presence of mitotic figures is in direct relation to the degree of malignant transformation. Tumors of the upper respiratory tract and airways have not been observed in response to inhalation exposure of asbestos or SVFs in rodents (IARC, 1987; 2002).
Mesothelioma has also been found in rodent carcinogenic bioassays of asbestos and SVFs (IARC, 1987; 2002. In inhalation studies in rats they are usually found at a lower incidence than lung cancer. Again, there does not appear to be a gender predisposition and the mesotheliomas in rodents typically occur late in life (after 21 months of age). They rarely are the cause of death. They grow by expansion, growing over the pleural surface. They typically do not invade the lung or other adjacent structures, although this has been observed. They usually present as multiple lesions on both sides of the lung and involve both the visceral and parietal pleura. Rarely, distant metastases have been observed. In inhalation studies, all of the major morphological types (tubulopapillary, sarcomatous and mixed) have been observed, although the tubulopapillary response is the predominate form. There is one exception to this and that is found in the inhalation study of erionite, where the sarcomatous type predominated, was highly invasive and the tumors were exceptionally lethal causing death in most of the rats by 15 months (Wagner et al., 1988). In contrast to inhalation, direct instillation into the pleural (Stanton et al., 1981) or peritoneal cavities (Pott et al., 1987) results in a preponderance of sarcomatous neoplasms, and in fact, it may be difficult to find mesothelial cells in many of the tumors, particularly after peritoneal injection. However, even in these studies, the mesotheliomas seldom invade local tissues or metastasize to other areas of the body.
The biological sequence of events in the development of mesothelioma in rodents also appears to have a series of progressive steps (Boorman et al., 1990). In inhalation studies, the first event that is observed is fibrosis in the pleura immediately subjacent to the mesothelial lining. This is multifocal in nature, possibly occurring more frequently in the interlobular pleura. In the few studies where the parietal pleura has been investigated (McConnell, et al., 1999), the initial change was found in the nonmuscular portion of the diaphragm and over the ribs (as compared to intercostal). The first indication of mesothelial change is found in these areas of pleural fibrosis. The mesothelial cells become cuboidal (as compared to a normal squamous morphology) and progress to focal hyperplasia of one to three cell layers thickness. The next step is the formation of papillary forms of growth and overgrowth of adjacent pleura. It is at this stage that mesothelioma is diagnosed. Pseudovacuolated tumor cells may be noted at this stage. Finally, the tumor evolves into the classical forms noted above. The course of events is somewhat different for instillation and injection studies. The initial response in the latter studies is inflammation, followed by a fibrogranulomatous reaction (assumed to be an attempt to wall off the fibers). A similar sequence of progression is assumed but results in a higher proportion of sarcomatous types of mesothelioma.
Pulmonary interstitial fibrosis (see below for description) is invariably found in studies where either asbestos or SVFs have caused either lung cancer or mesothelioma (Greim et al., 2001). However, there have been fiber studies where pulmonary fibrosis was observed without the development of fiber related neoplasms (McConnell, et al., 1994).
In vitro studies may not be of high value for predicting the carcinogenic potential of a given type of fiber, although they can give some incite into the difference between the carcinogenicity of long and short fibers. There are several reasons for why they may not as useful for predicting the carcinogenic activity of a given type. First, the fiber used is not subjected to physiological processes such as clearance and dissolution that are found in the lung. Also, the in vitro test systems use “fresh” fibers so do not typically take into account pathology attenuating changes in fibers that occur over time in the lung. Finally, the in vitro “dose” may have no relevance to the lung fiber burden. However, not withstanding this, in vitro methods are highly powerful tools for understanding fiber/cell interactions and mechanisms of toxicity/carcinogenicity (see Mossman for details).
Non-cancer effects: Animal models have also demonstrated many of the same pathological responses that are found in humans exposed to particulates (IARC, various volumes). The major noncancer endpoints that have been described in animals in experimental studies are phagocytosis, inflammation and pulmonary fibrosis. In regard to these endpoints, the rodent lung (and presumably other species) reacts to asbestos and SVFs as it would to any inhaled nonorganic foreign body that is not chemically toxic, e.g. beryllium. The lung can only react to such materials in a limited number of ways. In animals, if the particulate were deposited in the upper respiratory tract, one would assume that it would be possible for it to cause local irritation. However, this has not been observed in inhalation studies, even at high exposure levels. It is assumed that the resident time for such particles is brief, not allowing for a pathologic response. The mucous layer in these tissues is relatively thick compared to the size of the particulate and the methods of removal are quite efficient. The same is true for the major airways. In experimental animals the airways are intact and have not been compromised by other toxicants as in humans, e.g. smoking. Therefore, particulates deposited on these surfaces are again efficiently removed via the mucociliary escalator and are either swallowed or expectorated. In either case, the resident time in the body is relatively brief.
For a particulate to cause pathology in experimental animals after inhalation, it must reach the alveolar region of the lung. Particulate size dictates whether this happens or not. If the particle reaches terminal bronchiole it causes a foreign body reaction which is dictated by dose, particle (fiber) size and to some extent physical chemistry. The lungs’ initial response is an attempt to remove the offending substance. This is accomplished by resident macrophages. If the particle is of a size that the macrophage can engulf (phagocytize), it will be “captured and removed from the lung either by translocation to the airways or draining lymphatics. As the dose (number of particulates) increases, more macrophages are recruited. However, if the dose is too large for the number of available macrophages to remove, an “overload” situation develops which results in other pathologic events. Such events have been documented in animals both by histopathology and physiological tests (see Oberdorster for details). If the fiber is too large to be phagocytized and removed, i.e. longer than the size of the macrophage [~13 um diameter in rats and hamsters, monkeys ~15 um, and humans ~21 um diameter (Krombach et al., 1997)], the fiber cannot be removed unless it is broken into shorter lengths or dissolves (Maxim and McConnell, 2001). Both of the latter two phenomena have been observed with several SVFs (see below).
If the dose overwhelms the physiological pulmonary defenses or the fiber is too large to be removed, the initial series of events in animals occur at the junction of the terminal bronchioles and proximal alveolar duct (this is where most of the fibers are initially deposited - It should be noted that rodents do not have a respiratory bronchiole, as do humans). In addition to a stimulating the local macrophages, an influx of additional macrophages is recruited to the area. At this point, the local type II alveolar cells (in the proximal alveoli) undergo metaplasia to a cuboidal appearance and become hyperplastic. The resulting lesion has been termed “bronchiolization” because the change mimics the appearance of the terminal airways. Increased amounts of mucous production and sometimes inspissation of the material often accompany this. Coincident to the bronchiolization, microgranulomas are observed. These appear to form from a coalition of macrophages and fibroblasts. At this time the microgranulomas are restricted to the proximal portion of the alveolar duct, particularly along the alveolar duct ridge. With time and continued insult the process proceeds peripherally and becomes more apparent. If the offending fiber persists, collagen is laid down in the adjacent interstitium (presumably by direct invasion of the fiber into the epithelium and interstitium). At this time the lesion is referred to as interstitial fibrosis. In rodent studies, the fibrotic areas are initially focal and widely disseminated. But, if the insult persists or the dose is high enough, fibrosis becomes more widespread. Various schemes have been developed to describe these events and grade them as to their severity for comparative purposes (McConnell et al., 1984: 2002). There is one notable difference between the qualitative appearance of the lesions produced by asbestos and SVFs in animals. Neutrophils are often a prominent part of the inflammatory reaction with asbestos, especially with amphiboles, while they are rarely found in studies of SVFs, even at doses that produce fibrosis. The inflammatory reaction can also be documented and quantified by using the results of pulmonary lavage studies (see Oberdorster).
Stop studies (where exposure is stopped and the animals are observed during a nonexposed recovery period) have proved useful for determining the reversibility of the above lesions. Such studies have clearly shown that the initial changes (macrophage response and bronchiolization) are totally reversible with most SVFs and to some degree with asbestos. Microgranulomas become less apparent and early fibrosis is also, to some degree, resolvable, at least with SVFs. In rodents, studies have demonstrated that fibrosis, even with asbestos, is not particularly progressive, once the exposure ceases.
While there is no exact correlate for pleural plaques in animals, localized acellular fibrotic changes reminiscent of this lesion in humans have been observed, albeit on a much smaller scale. The qualitative changes in the pleura are somewhat different than in the lung. Macrophages and inflammatory cells are almost totally absent in the pleural response. Lavage studies have not been conducted with pleural instillation or peritoneal injection studies so it is not known if the same events occur with these routes of exposure of exposure. Animal inhalation studies also suggest that fibers need to be present in the pleura for pathologic events to occur.
In vitro studies of mesothelial cells have been conducted using both human and animal cells. These have been primarily designed to study the mechanisms of carcinogenicity (see Mossman).
Irritant effects: While there is evidence of dermal and ocular irritation of humans as a response to exposure to asbestos and SVFs, no such evidence has been observed in animals. Histopathological studies of the nasal cavity in rodents exposed via inhalation have not shown any evidence of pathology, although an increased mucous response could be missed with standard histopathology techniques. Similarly, ingestion studies in rats and hamsters of asbestos did not reveal any irritation of the alimentary tract (ATSDR, in press).
In vitro studies on the irritant effects of either asbestos or SVFs in animals have not been reported.
Association between fiber length and fiber-like toxicity: There are numerous animal studies that demonstrate the influence of fiber length and pathogenicity/carcinogenicity. The early studies by using intrapleural implantation/instillation (Stanton et al., 1981) and intraperitoneal injection (Pott et al., 1976) in rats clearly show a direct relationship between fiber size and carcinogenic activity. The longer the fiber, the more carcinogenic it was in these studies. These same studies provided the basis for the hypothesis that short fibers, i.e. shorter than 8 um in length may not represent a significant carcinogenic risk. However, the same investigations, particularly the intraperitoneal studies also demonstrated that if the dose was high enough even so-called “innocuous” particulates, e.g. titanium dioxide, caused the induction of peritoneal mesotheliomas, albeit at a lower incidence than long fibers. Additionally, the latter studies also demonstrated that if even long fibers, e.g. wollastonite and some SVFs, were not carcinogenic if they were not biopersistent in the peritoneal cavity. There have been a few inhalation studies have been conducted to study the influence of the fiber length on the pathology of asbestos, and all have been persuasive for showing that short fibers are not carcinogenic. This has been demonstrated for chrysotile (Davis and Jones, 1988; Ilgren, 1998; Wagner et al., 1980), amosite (Davis et al., 1987; 1986) and crocidolite (Davis et al., 1978; Wagner et al., 1984).
Other circumstantial evidence for considering fiber length as being critical to the carcinogenic potential of fibers is provided by the observation that amorphous silica has been shown to be noncarcinogenic in several inhalation studies in rats, while some types of glass fibers of similar chemistry have shown to have carcinogenic activity (IARC, 1987). In fact, amorphous silica has been used as a “negative control” in rodent inhalation studies. A final piece of evidence for the importance of fiber length for the carcinogenic of asbestos and SVFs is found in the hilar lymph nodes that drain the lungs of animals exposed via inhalation to both asbestos and SVFs. These lymph nodes are literally filled with macrophages containing short fibers and fiber fragments with no evidence of pathology or neoplastic change in either the lymph nodes or adjacent tissues.
To summarize studies in animals of short fibers and nonfibrous particulates have shown that both are potentially carcinogenic if they are introduced into a confined cavity, e.g. pleural or peritoneal, at sufficiently high doses. But the same studies clearly show that the carcinogenic potential is definitely less with fibers of the same type that are longer. Inhalation studies have clearly shown that short fibers have not caused cancer in animals. The other part of the equation that needs to be considered is the influence of pulmonary clearance and biopersistence on the carcinogenic potential of particulates. As noted above, even long fibers are not carcinogenic in animals unless they are biopersistent in the animal.
There are only a few in vitro studies that address this subject but those that have clearly show a relationship between fiber length and genetic damage. For example, in a study of Chinese hamster ovary cells (CHO) short amosite failed did not cause chromosomal aberrations while long fiber amosite did (Donaldson and Golyasnya, 1995). See Mossman and others for other studies.
Thresholds of toxic action: There have been very few inhalation studies in animals of either asbestos or SVFs to assess a carcinogenic dose response. It needs to be remembered that to assess a carcinogenic dose response, one must have a multidose study that shows a carcinogenic response. Most asbestos and SVF studies were designed to address the carcinogenic potential of the fiber, not dose response. The only multi-dose inhalation study of asbestos used amosite in hamsters (McConnell et al., 1995). In that study, there was a definite dose-related response with regard to both nonneoplastic (macrophage response, pulmonary fibrosis, etc.) and carcinogenic activity (mesothelioma). Unfortunately, the potential lung cancer response could not be assessed because hamsters do not develop pulmonary tumors with particulates. There are a few inhalation studies of SVFs that address dose response. The only one that was positive for cancer involved refractory ceramic fibers in rats (Mast et al., 1995). In that study there was a clear dose response for both cancer and noncancer endpoints and a no-effect level. There are a few other multidose studies in rats using various types of SVFs, but since none showed carcinogenic activity, one can only evaluate the dose response for noncancer endpoints (Hesterberg et al., 1996). Again, there was evidence in these studies of a dose-related change in the endpoints showing recognizable change. The “stop-studies” in many of these inhalation studies (both asbestos and SVFs) provide evidence for a dose response for noncancer endpoints. However, the number of animals evaluated in the “stop studies” is too small to address a cancer dose response. The only study in primates that addresses a potential threshold of action was with chrysotile asbestos (Patek et al., 1985). In this study, monkeys were exposed to chrysotile asbestos at an exposure level of 1 mg/m3 (0.8 f/cc >5 um length) for 18 months. Ten months following the last exposure, lung biopsies were taken and evaluated for fiber burden and histopathology. There was no evidence of pathology although a few asbestos bodies were observed in the lung. The monkeys were then held unexposed for an additional ~11 years at which time they were subjected to necropsy examination and the lungs for histopathology examination. Again, there was no evidence of pulmonary pathology and the number of asbestos bodies had decreased (not reported — personal observation).
In summary, the totality of available data suggests that there is a dose-response for both neoplastic and nonneoplastic endpoints in animals and there is a no effect level for both asbestos and SVFs. One attempt at deciding if a given exposure in animals is potentially carcinogenic involves the use of noncancer endpoints. In this scheme it was assumed that a dose that caused pulmonary fibrosis could also represent an exposure that was potentially carcinogenic in animals. This was because no animal study has ever produced cancer in the absence of fibrosis. The next assumption was that since no inhalation study had ever shown fibrosis in the absence of inflammation, one could assume that an exposure that didn’t result in inflammation would not reasonably be expected to be carcinogenic. The endpoint chosen for assessing inflammation was the presence of inflammatory cells over background in bronchoalveolar lavage (BAL) fluid after a 90-day inhalation exposure. Therefore, if one did not find an increase in inflammatory cells in BAL fluid, one could chose this exposure as a no-effect threshold.
It is reasonable to expect that in vitro studies could shed light on the dose response of both asbestos and SVFs. While these types of studies are primarily designed to capture and elucidate specific mechanisms of toxicity and carcinogenicity, there may be insights into dose response that could help in establishing thresholds of effect. One such study showed that short fiber amosite did not cause inflammation, while long amosite did. The only draw backs to and in vitro approach is that these techniques do no take lung clearance phenomena into consideration and fibers that are not biopersistent in the lung might not be differentiated from biopersistent ones because of the short time frame of the in vitro studies.
References
ATSDR, (in press) Toxicological Profile for Asbestos. DHHS, Agency for Toxic Substances and Disease Registry.
ATSDR, (in press) Toxicological Profile for Synthetic Vitreous Fibers. DHHS, Agency for Toxic Substances and Disease Registry.
Boorman et al. 1990. Pathology of the Fischer Rat. Chap. 6. Peritoneum, Retroperitoneum, Mesentery, and Abdominal Cavity.
Boorman et al. 1990. Pathology of the Fischer Rat. Chap. 21. Lung.
Davis, J.M. et al. 1978. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br. J. Cancer. 37:673-688.
Davis, J.M. et al. 1986. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br. J. Exp. Pathol. 67:415-430.
Davis, J.M. and Jones, A.D. 1988. Comparisons of the pathogenicity of long and short fibres of chrysotile asbestos in rats. Br. J. Exp. Pathol. 69:717-737.
Donaldson, K. and Golyasnya, N. 1995. Cytogenic and pathogenic effects of long and short amosite asbestos. J. Pathol. 177:303-307.
Greim, H. et al. 2001. Toxicity of febers and particles — Report of the workshop held in Munich, Germany, 26-27 October 2000. Inhal. Toxicol. 13:737-754.
Hesterberg, T.W. et al. 1996. Use of lung toxicity and lung particle clearance to estimate the maximum tolerated dose (MTD) for a fiber glass chronic inhalation study in the rat. Fund. Appl. Toxicol. 32:31-44.
IARC, 1987. Asbestos and certain asbestos compounds. In: IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans. Suppl. 7.
IARC, 1987. Man-Made Vitreous Fibers. In: IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans. Vol. 81.
Ilgren, E and Chatfield, E. 1998. Coalinga fibre — A short, amphibole-free chrysotile. Part 2: Evidence for lack of tumourigenic activity. Indoor Built Environment. 7:18-31.
Krombach, et al. 1997. Cell size of alveolar macrophages: An interspecies comparison. Environ. Health Persp. 105(Suppl 5):1261-1263
Mast, R.W., et al. 1995. A multiple dose chronic inhalation toxicity study of size-separated kaolin refractory ceramic fiber (RCF) in male Fischer 344 rats. Inhalation Toxicol. 7:469-502.
Maxim, L.D. and McConnell, E.E. 2001. Interspecies comparisons of the toxicity of asbestos and synthetic vitreous fibers: A weight-of-the-evidence approach. Reg. Toxicol. Pharmacol. 33:1-24.
McClellan, R.O., et al. 1992. Approaches to Evaluating the Toxicity and Carcinogenicity of Man-Made Fibers: Summary of a Workshop Held November 11-13, 1991, Durham, North Carolina. Reg. Toxicol. Pharm., 16:321-364.
McConnell, E.E., et al. 1984. A comparative study of the fibrogenic and carcinogenic effects of UICC Canadian chrysotile B asbestos and glass microfibre (JM 100). In: Biological Effects of Man-made Mineral Fibres. World Health Organization, pp. 234-252.
McConnell, E.E., et al. 1994. Chronic inhalation study of size-separated rock and slag wool insulation fibers in Fischer 344/N rats. Inhalation Toxicol., 6:571-614.
McConnell, E.E. 1995. Advantages and limitations of in vivo screening tests. Ann. Occup. Hyg. 39:727-735.
McConnell, E.E., et al. 1995. Chronic inhalation toxicity of a kaolin based refractory ceramic fiber (RCF) in Syrian golden hamsters. Inhalation Toxicol. 7:503-532.
McConnell, E.E., et al. 1999. Studies on the inhalation toxicology of two fiberglasses and amosite asbestos in the Syrian golden hamster. Part 2. Results of chronic exposure. Inhal. Toxicol. 11:785-836.
McConnell, E.E. and J.M.G. Davis. 2002. Quantification of fibrosis in the lungs of rats using a morphometric method. Inhalation Toxicol. 14:101-110.
Platek, S.F., et al. 1985. Chronic irritation of short asbestos fibers. Fund. Appl. Toxicol. 8:327-340.
Pott, F. et al., 1976. Results of animal experiments concerning the carcinogenic effect of fibrous dusts and their interpretation with regard to the carcinogenesis in humans. Abl. Bakteriol. Orig. B. 162:467-505.
Pott, F. et al. 1987. Carcinogenicity studies of fibres, metal compounds, and some other dusts in rats. Exp. Pathol. 82-129.
Pott, F. et al. 1994. Lung tumours in rats after intratracheal instillation of dusts. Ann. Occup. Hyg. 38:357-363.
Stanton, M.F., et al. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J. natl Cancer Inst. 67: 965-975.
Wagner, J.C., et al. 1974. The effects of the inhaltion of asbestos in rats. Br. J. Cancer 29:252-269.
Wagner, J.C. et al. 1980. The comparative effects of three chrysotiles by injection and inhalation in rats. IARC Sci. Publ. 30:363-372.
Wagner, J.C. et al., 1984. The effect of fibre size on the in vivo activity
of UICC crocidolite. Br. J. Cancer 49(4):453-458
Dr. Mossman’s Post-Meeting Comments
ATSDR Fibers Panel Mechanisms of Short Fiber Toxicity
There appears to be a striking difference in the pathogenicity of respirable fibers directly related to fiber length, with fibers below approximately 5 microns in length being less hazardous for the development of cancers or pulmonary fibrosis. This prompts the questions: What are the observed mechanisms of long fiber toxicity? Does composition matter? Are short (<5 microns in length) fibers pathogenic? If so, what are the mechanisms of their toxicity?
One hypothesis is that long fiber effects are related to increased generation of oxidants; reviewed in Kinnula, 1999; Hansen and Mossman, 1987). It has been shown that reactive oxygen species (ROS) and reactive nitrogen species (RNS) are generated by asbestos fibers spontaneously in cell-free systems, cells in culture, and lung tissue in vivo. A primary step in response to asbestos fiber challenge to a number of cell types is superoxide anion release from cells which have attempted to phagocytize long fibers whereas short fibers are encapsulated in phagolysosomes, often without visible damage to cells. Superoxide, however, can be further dismutated to hydrogen peroxide which can generate the reactive hydroxyl radical, catalyzed by iron vs. the Fenton reaction. Alternatively, superoxide can react with nitric oxide to form peroxynitrite that is associated with inflammation and lung injury. Asbestos stimulates the release of ROS and induces oxidants intracellularly in both inflammatory cell types (Hansen and Mossman, 1987; Goodglick and Kane, 1986; 1990) and target cells (Xu et al., 2002). Moreover, indirect evidence for oxidant stress by asbestos is indicated by elevations of antioxidant enzymes in cells in culture and lung tissue after inhalation of crocidolite asbestos (Janssen et al, 1992, 1994b). In human mesothelial cells, these increases were not observed with exposures to polystyrene beads, or riebeckite, a chemically similar nonfibrous analog of crocidolite (Janssen et al., 1994b). The role of oxidants by crocidolite asbestos in causation of inflammation and fibrosis has been confirmed in rodent inhalation studies (Mossman et al., 1990), and supports the central dogma that asbestos fibers activate transcription factors and early response genes involved in proliferation and inflammation by generating ROS via “frustrated phagocytosis” (reviewed in Manning et al., 2002).
Several papers show that “frustrated phagocytosis” and oxidant production occur selectively in response to long vs. short fibers of asbestos or glass. A study of luciginen-dependent chemiluminescence (CL) in human monocytes found a strong correlation between superoxide release and fiber lengths from 6 to 20 microns. All samples of fibers except wollastonite induced CL release in a dose-dependent manner. Superoxide release was non-specific for the compositional type of fiber, and fibers with lengths below 7 microns were only weakly active. In studies by Blake et al. (1998), CL induction after zymosan stimulation and LDH release, a measure of lytic cell death, were measured in Manville Code 100 (JM-100) fiber challenged rat alveolar macrophages. A novel feature of this study was the use of fibers carefully sized to average lengths of 33, 17, 7, 4, and 3 microns. The greatest toxicity was seen with the longer fibers which had multiple macrophages attached along the surface, indicating that incomplete phagocytosis was associated with toxicity. These studies reinforce the many experiments in the literature showing that long fibers are more toxic than shorter fibers in a number of cell types, i.e., Goodglick and Kane, 1990.
Increased fiber length has also been linked to activation of transcription factors and cytokines. For example, Tumor Necrosis Factor-alpha (TNF) is a cytokine involved in inflammation and fibrosis. In a study by Ye et al. (1999), glass fibers with lengths of 6.5 +/- 2.7 microns and 16.7+/-10.6 microns were used to challenge a mouse macrophage cell line. Glass fibers stimulated TNF production and caused Nuclear Factor- kB (NF- kB) activation, a process involving ROS. Long fibers were more potent than short fibers which were effectively engulfed by macrophages. Short fiber-induced TNF and TNF gene promoter activation was on the order of one-third to one-half of long fibers. In another study (Cheng et al., 1999), crocidolite asbestos caused parallel increases in TNF production in macrophages in a dose-dependent manner, without cytotoxicity at the optimum stimulating condition. Titanium oxide dust was without effect. TNF production may also be linked to inflammation by asbestos, and it has been shown that injection of long vs. short amosite fibers intraperitoneally results in inflammation and macrophage activation related to the proportion of long fibers (Donaldson et al., 1989).
Another pathway leading to activation of protooncogenes (fos/jun) that comprise the Activator Protein-1 transcription factor is the Mitogen Activated Protein Kinase (MAPK) cascades, consisting of c-jun-N-terminal amino kinases (JNKs), Extracellular Signal Regulated Kinases (ERKs) and p38 kinases. In studies by Ye et al. (2001) using macrophages, long glass fibers were more potent than short fibers in activating MAPK which led to activation of c-Jun and the TNF promoter. Studies by Zanella et al. (1996) explored the stimulation of ERKs in mesothelial cells, and found increases with crocidolite and chrysotile asbestos , but not with the nonfibrous analogs, riebeckite or antigorite. Similarly, elevations in c-fos and c-jun expression were seen with asbestos fibers and erionite in mesothelial cells, but were not induced by a variety of particulates, MMVF-10 or RCF-1 fibers at comparable concentrations (Janssen et al., 1994a). Long fibers of crocidolite (> 60 microns) were selectively associated with phosphorylation of the Epidermal Growth Factor receptor in human mesothelial cells (Pache et al., 1997), an event not occurring with MMVF-10 or particles. In general, pathogenic dusts such as asbestos or silica, produce a variety of cytokines from cells and activate a number of transcription factors through ROS or RNS (Mossman and Churg, 1998; Churg et al., 2000).
Another ramification of transcription factor activation is cell proliferation. Mechanistic studies using target cells in culture or tracheal explants have shown that long fibers are more toxic and more apt to cause cell proliferation than short fibers (Brown et al., 1986; Wright et al., 1986; Marsh and Mossman, 1988; Sesko and Mossman, 1989; Woodworth et al., 1983). These events may be coupled, as compensatory hyperplasia may result from initial epithelial cell injury. In studies by Woodworth et al. (1983), epithelial proliferation and squamous metaplasia were observed with various types of fibers including glass and attapulgite, but not with nonfibrous analogs of asbestos, i.e. riebeckite and antigorite and other particles.
An intratracheal model in rats using long (>2.5 microns) and short crocidolite asbestos after intratracheal instillation has yielded some mechanistic information on the differential effects of long vs. short fibers (Adamson and Bowden 1987a, b; 1990). These studies suggest that the increased fibrogenic response to long fibers may be due to selective increases in cell proliferation. In addition, both long and short asbestos fibers cause alveolar macrophages to secrete fibrogenic cytokines, but interstitial fibroblasts exposed to short asbestos fibers do not respond to these cytokines.
Surfactant adsorption may be a mechanism whereby reactive particles or fibers are rendered inactive or nonpathogenic. To determine the effect of surfactant adsorption on chrysotile genotoxicity using an assay for micronucleus induction in Chinese hamster lung cells (V79) (Lu et al., 1994), two lengths of chrysotile fibers were used with and without pretreatment with DPPC, i.e. NIEHS intermediate (65%> 10 microns) and short (98% < 10 micron) fibers. The longer fibers were most active, and DPPC treatment diminished the activity approximately 15%. The maximum activity of the short fiber sample was 70% of the activity of the non-treated intermediate, and the DPPC-treated short fibers expressed about 45% of the activity of the untreated. That is, DPPC did not fully suppress the activity of the fibers, but had a much more pronounced effect on the short fibers. One possibility is that the partial suppression of genotoxicity reflects suppression of a component of toxicity by surfactant on the mineral surface. Thus, short fiber genotoxicity, as reported here, may reflect a combination of mineral surface functional groups which direct membranolytic activity and can be modulated by interactions with components of the pulmonary surfactant system as well as phagocytosis-associated ROS.
In conclusion, studies summarized above show decreased or no effects of short fibers and nonfibrous analogs of asbestos in a number of bioassays. The effects of long glass and asbestos fibers may be comparable in some studies. However, the duration of these short-term assays may be too short to reflect important solubility changes occurring in lung over time.
References
Adamson IYR, DH Bowden. (1987a) Response of mouse lung to crocidolite asbestos. 1. Minimal fibrotic reaction to short fibers. Journal of Pathology 152:99-107.
Adamson IYR, DH Bowden. (1987b) Response of mouse lung to crocidolite asbestos. 2. Pulmonary fibrosis after long fibers. Journal of Pathology 142:109-117.
Adamson IYR, DH Bowden. (1990) Pulmonary reaction to long and short asbestos fibers is independent of fibroblast growth factor production by alveolar macrophages. American Journal of Pathology 137:523-529.
Blake T, V Castranova, D Schwegler-Berry, P Baron, GJ Deye, C Li, W Jones. (1998) Effect of fiber length on glass microfiber cytotoxicity. Journal of Toxicology and Environmental Health 54:243-259.
Brown GM, H Cowie, JMG Davis, K Donaldson. (1986) In vitro assays for detecting carcinogenic mineral fibres: A comparison of two assays and the role of fibre size. Carcinogenesis 7(12):1971-1974.
Cheng N, X Shi, J Ye, V Castranova, F Chen, SS Leonard, V Vallyathan, Y Rojanasakul. (1999) Role of transcription factor NF-kappaB in asbestos-induced TNFalpha response from macrophages. Experimental and Molecular Pathology 66(3):201-210.
Churg A, J Wright, B Gilks, J Dai. (2000) Pathogenesis of fibrosis produced by asbestos and man-made mineral fibers: what makes a fiber fibrogenic? Inhalation Toxicology 12(Suppl 3):15-26.
Donaldson K, GM Brown, DM Brown, RE Bolton, JMG Davis. (1989) Inflammation generating potential of long and short fibre amosite asbestos samples. British Journal of Industrial Medicine 46:271-276.
Goodglick LA, AB Kane (1986) Role of reactive oxygen metabolites in crocidolite asbestos toxicity to mouse macrophages. Cancer Research 46:5558-5566.
Goodglick LA, AB Kane. (1990) Cytotoxicity of long and short crocidolite asbestos fibers in vitro and in vivo. Cancer Research 50:5153-5163.
Hansen K, BT Mossman. (1987) Generation of superoxide from alveolar macrophages exposed to asbestiform and nonfibrous particles. Cancer Research 47:1681-1686.
Janssen YMW, JP Marsh, MP Absher, D Hemenway, PM Vacek, KO Leslie, PJA Borm, BT Mossman. (1992) Expression of antioxidant enzymes in rat lungs after inhalation of asbestos or silica. Journal of Biological Chemistry 267:10625-10630.
Janssen YMW, NH Heintz, JP Marsh, PJA Borm, BT Mossman. (1994a) Induction of c-fos and c-jun proto-oncogenes in target cells of the lung and pleura by carcinogenic fibers. Am. J. Respiratory Cell and Molecular Biology 11:522-530.
Janssen YMW, JP Marsh, MP Absher, E Gabrielson, PJA Borm, K Driscoll, BT Mossman. (1994b) Oxidant stress responses in human pleural mesothelial cells exposed to asbestos. American Journal of Respiratory and Critical Care Medicine 149:795-802.
Kinnula VL. (1999) Oxidant and antioxidant mechanisms of lung disease caused by asbestos fibres. European Respiratory Journal 14:706-716.
Lu J, MJ Keane, T Ong, and WE Wallace. (1994) In vitro genotoxicity studies of chrysotile asbestos fibers dispersed in simulated pulmonary surfactant. Mutation Research 320(4):253-259.
Manning CB, V Vallyathan, BT Mossman (2002) Diseases caused by asbestos: mechanisms of injury and disease development. In: JE Talmadge and T Hugli, eds. Int. Immunopharmacology; (MI Luster and MH Karol, guest eds); Vol. 2, pp. 191-200.
Marsh JP, BT Mossman. (1988) Mechanisms of induction of ornithine decarboxylase activity in tracheal epithelial cells by asbestiform minerals. Cancer Research 48:709-714.
Mossman BT, JP Marsh, A Sesko, S Hill, MA Shatos, J Doherty, J Petruska, KB Adler, D Hemenway, R Mickey, P Vacek, E Kagan (1990) Inhibition of lung injury, inflammation and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. American Review of Respiratory Diseases 141:1266-1271,
Mossman BT, A Churg. (1998) State-of-the-Art. Mechanisms in the pathogenesis of asbestosis and silicosis. American Journal of Respiratory and Critical Care Medicine 157:1666-1680
Pache JC, YMW Janssen, ES Walsh, TR Quinlan, CL Zanella, RB Low, DJ Taatjes, BT Mossman. (1998) Increased epidermal growth factor-receptor protein in a human mesothelial cell line in response to long asbestos fibers. American Journal of Pathology 152:333-340.
Sesko A, BT Mossman. (1989) Sensitivity of hamster tracheal epithelial cells to asbestiform minerals is modulated by serum and by transforming growth factor, beta 1. Cancer Research 49:2743-2749.
Woodworth CD, BT Mossman, JE Craighead. (1983) Induction of squamous metaplasia in organ cultures of hamster trachea by naturally occurring and synthetic fibers. Cancer Research 43:4906-4912.
Wright A, H Cowie, IP Gormley, JMG Davis. (1986) The in vitro cytotoxicity of asbestos fibers. I. P388D1 cells. American Journal of Industrial Medicine 9(4):371-384.
Xu A, H Zhou, D Zengliang Yu, TK Hei. (2002) Mechanisms of the genotoxicity of crocidolite in mammalian cells: implication from mutation patterns induced by reactive oxygen species. Environmental Health Perspectives 110:1003-1008.
Ye J, X Shi, W Jones, Y Rojanasakul, N Cheng, D Schwegler-Berry, P Baron, GJ Deye, C Li, V Castranova. (1999) Critical role of glass fiber length in TNF-alpha production and transcription factor activation in macrophages. American Journal of Physiology 276(3 Pt 1):L426-L434.
Ye J, P Zeidler, SH Young, A Martinez, VA Robinson, W Jones, P Baron, X Shi, and V Castranova. (2001) Activation of mitogen-activated protein kinase p38 and extracellular signal-regulated kinase is involved in glass fiber-induced tumor necrosis factor-alpha production in macrophages. Journal of Biological Chemistry 276:5360-5367.
Zanella CL, J Posada, TR Tritton, BT Mossman. (1996) Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Research 56:5334-5338.
Dr. Oberdörster’s Post-Meeting Comments
When responding to the charge questions in Topic Area 1 (physiological fate of asbestos and SVF fibers less than 5 micrometers in length), Dr. Oberdörster gave a brief presentation to the panel. He asked that a copy of the overheads from this presentation be included in this appendix of the report. A copy of the overheads Dr. Oberdörster prepared for the meeting follow, including some overheads that were not shown at the meeting due to time constraints.
Dr. Oberdörster also provided an additional comment not mentioned at the expert panel meeting. He noted that the panelists overlooked an important concept of short fiber toxicity which involves an increased retention in the lung of short fibers in people (e.g., smokers) who have disturbed alveolar macrophage mediated lung clearance. These people, he noted, can experience a marked increase in short fiber retention and thereby increase the potential for fiber toxicity significantly. Long fibers are reportedly not affected to the same degree as short fibers, as was described in a paper by Churg (“Effects of cigarette smoke on the clearance of short asbestos fibres from the lung and a comparison with the clearance of long asbestos fibres,” International Journal of Experimental Pathology 73(3): 287-297, 1992).
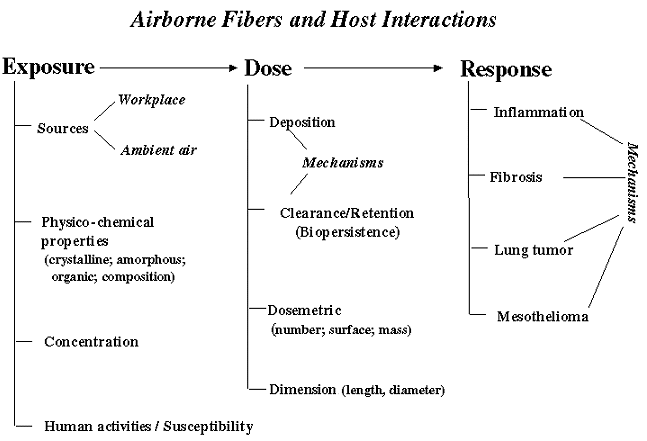
Main Depostion Mechanisms of Inhaled Fibers
- Impaction (abrupt directional changes)
- Sedimentation (gravitational settlings)
- Diffusion (Brownian motion)
- Interception (long fibers)
From: Asgharian and Yu, 1988
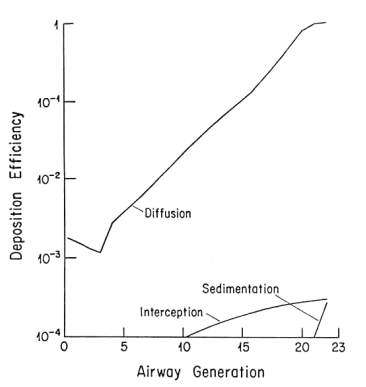
Deposition Efficiency of Fibers in Different Generations of the Weibel Lung Model at a Flow Rate of 375 cm3 /sec for dem =0.01µm and Unit Density.
From: Asgharian and Yu, 1988
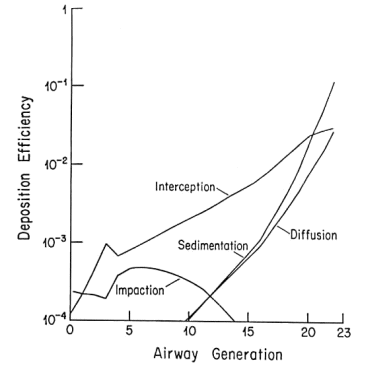
Deposition Efficiency of Fibers in Different Generations of the Weibel Lung Model at a Flow Rate of 375 cm3 /sec for dem = 1 µm and Unit Density.
From: Asgharian and Yu, 1988
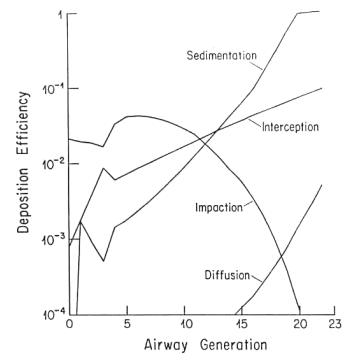
Deposition Efficiency of Fibers in Different Generations of the Weibel Lung
Model at a Flow Rate of 375 cm3 /sec for dem = 10 µm
and Unit Density.
Predicted deposition of fibers in human
extrathoracic airways
(after Yu, 1990)
Mouth Breathing
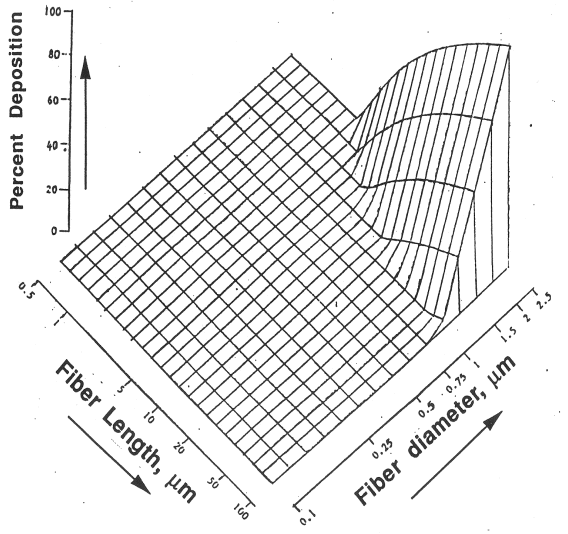
Nose Breathing
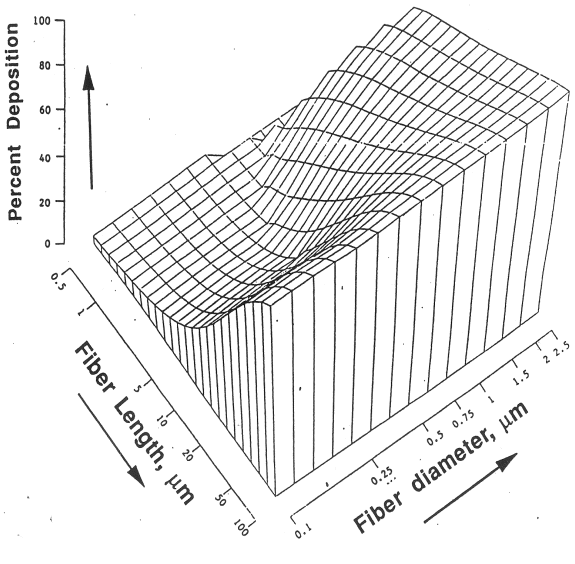
Effective filtration of long fibers by nose
Asgharian and Yu, 1989
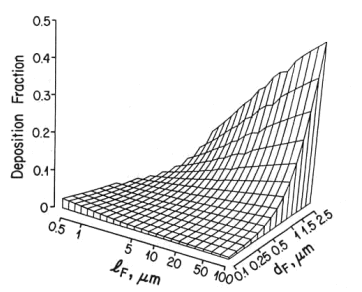
Fig 3. Tracheobronchial deposition via trachea with no interceptional deposition
at a lung volume of 10.78 cm3, a tidal volume of 1.68 cm3,
a breathing frequency of 98 cycles min -1 and p=3.37 g cm -3.
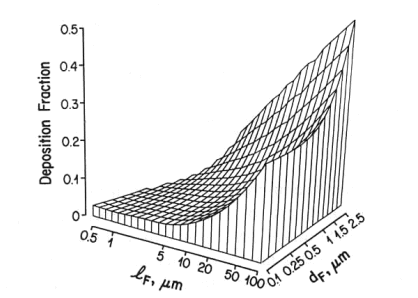
Fig 4. Tracheobronchial deposition via trachea at a lung volume of 10.78 cm3, a tidal volume of 1.68 cm3, a breathing frequency of 98 cycles min -1 and p=3.37 g cm -3.
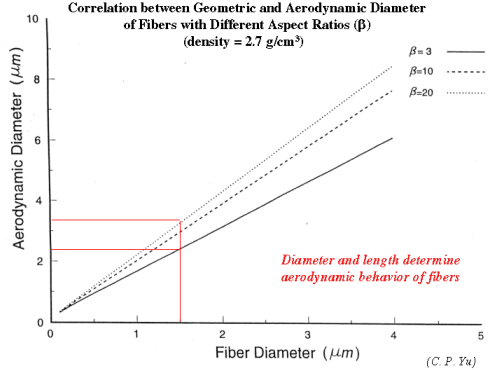
Repirability of Inhaled Fibers
Deposition in Alveolar Region
Long fibers are poorly respirable in rats
Retained Dose |
= |
Deposited Dose |
- |
Amount Cleared |
(Retention |
= |
Deposition |
- |
Clearance) |
Physiological Clearance Mechanisms of Deposited Fibers
- mucociliary movement (nose; tracheobronchial region)
- alveolar macrophages* (size limitation)
- interstitial translocation (pleura)
- lymphatic clearance (size limitation)
In addition: Clearance is determined by fiber specific physicochemical processes
Together, these mechanisms define the Biopersistence of a Fiber
| *normal AM-mediated clearance: | T1/2 rat ~70 days |
| T1/2 human ~400 - 700 days |
Pathogenicity and Fiber Length:
The Role of Alveolar Macrophage (AM) Size
| Hypothesis: | Phagocytizable fibers | efficient clearance and prevention of target cell interaction |
Average AM Diameters:
| Rat: | 10.5 — 13 µm | Crapo et al., 1983; Lum et al., 1983, Stone et al., 1992; | |
| Human: | 14 — 21 µm | Sebring and Lehnert, 1992; Krombach et al., 1997 |
| For cancer | number of fibers longer than 20 µm | |
| For non-cancer | all fibers (but: also impact for tumors!) |
Size Dependent Lymphatic Clearance of Amosite Asbestos
Study: Intrabronchial instillation of amosite in dogs, followed by
Analysis of mediastinal lymph node and of lymph collected from
Right Lymph Duct
| Max. Diameter | Max. Length |
|
| Lymph node | 0.5 µm | 16 µm |
| Post-nodal lymph | 0.5 µm | 9 µm |
(Oberdörster et al., 1988)
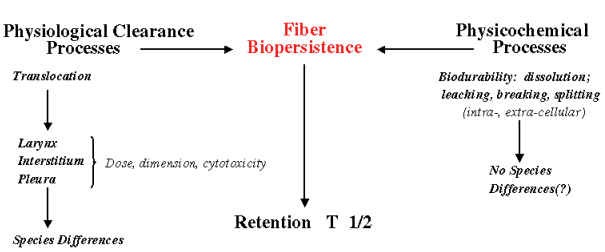
Biopersistence = Biodurability + Physiological Clearance
(Oberdörster, 1996)
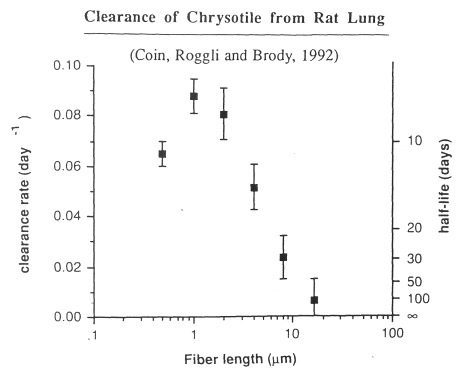
From: Timbrell, Inhaled Particles V, 1982
FIG. 8. Bivariate distribution of fibres in Paakkila bagging section.
From: Timbrell, Inhaled Particles V, 1982
FIG. 10. Bivariate presentation of fibre retention
Fiber Dosimetry Following RCF-1 Exposure
(Gelzleichter et al., 1996)
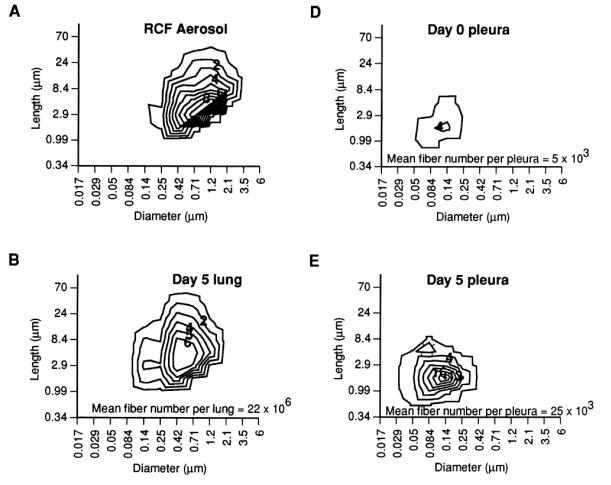
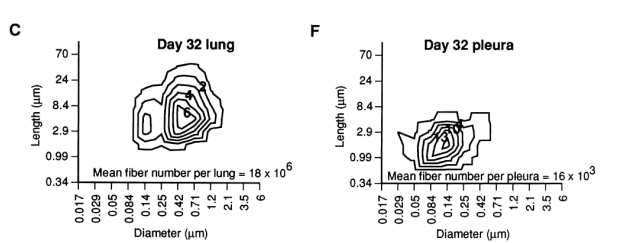
FIG 2. Relative size distribution of fibers isolated from (A) aerosol, (B) Day 5 lung, (C) Day 32 lung, (D) Day 0 pleura, (E) Day 5 pleura, and (F) Day 32 pleura. Isobars were calculated based on histogram analysis of the aerosol cloud and fiber burden data (average of six rats). Median bin values are shown on the x and y-axes. Pulmonary and pleural fiber burdens were normalized to Day 5 data with areas under the curve equal to 100 on Day 5.
From: Timbrell et al., Inhaled Particles VI, 1988
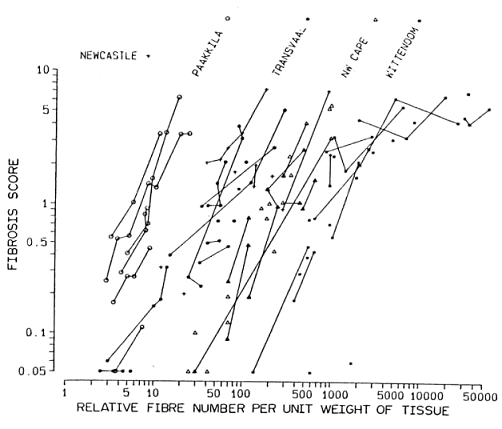
FIG 5. Relationships when the parameter of fibre quantity is number.
From: Timbrell et al., Inhaled Particles VI, 1988
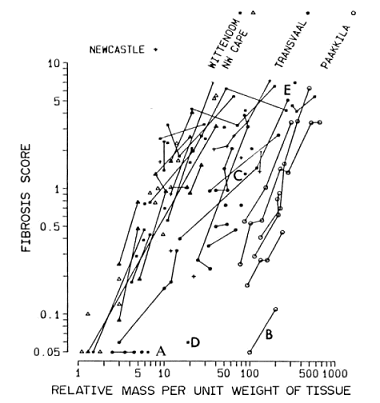
FIG. 3. Relationships when the parameter of fibre quantity is mass. Linked data points relate to tissue specimens from the same subject.
From: Timbrell et al., Inhaled Particles VI, 1988
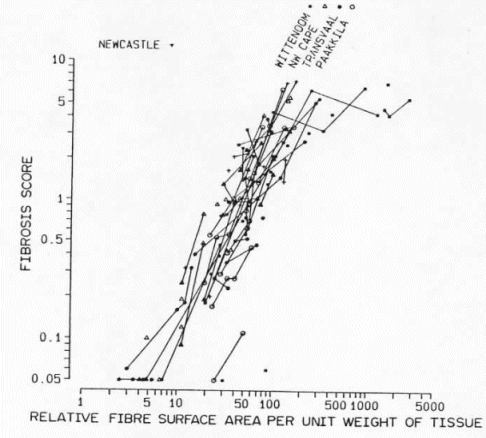
FIG. 4. Relationships when the parameter of fibre quantity is surface area.
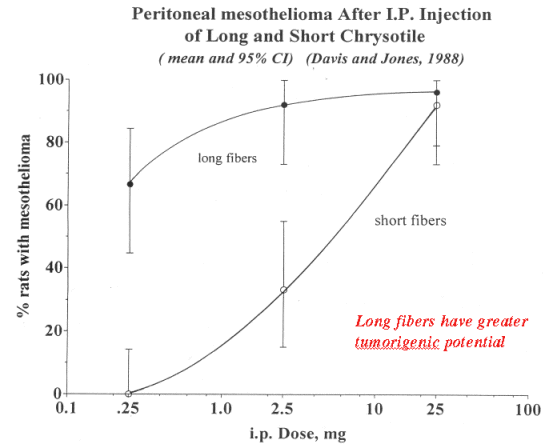
Mixed Dust Exposures in Rats (Davis et al., 1991)
Fractional Deposition of Inhaled Particles in the Human
Respiratory Tract
(ICRP Model, 1994; Nose Breathing)
A= Alveolar; TB = Tracheobronchial; NPL = Nasal, Pharyngeal, Laryngeal
Classification
| Natural Fibers | Man-made Fibers |
||||
| Asbestos | Asbestiform | Vitreous | Crystalline | Organic | |
| Serpentine | Fibrous clays | Glass wool | Alumina | Para-aramid | |
| chrysotile | polygorskite (attapulgite) | Glass filaments | Graphite | Cellulose | |
| sepiolite | Rockwool | Potassium titanante |
Carbon | ||
| Slagwool | Silicon carbide | ||||
| Amphiboles | Other Fibrous Silicates | Ceramic fibers | Sodium aluminum carbonate |
||
| actinolite | wollastonite | Synthetic zeolites | |||
| amosite | nemalite (fibrous brucite) | ||||
| anthophyllite | talc | ||||
| crocidolite | zeolites: | mordenite | |||
| tremolite | erionite | ||||
Mechanisms of Fiber Carcinogenesis
Kane, 1996
1. Fibers generate free radicals that damage DNA
— direct generation of ROS and damage of DNA in cell free system
— catalyse oxidation of PAH to free radical
— activate phagocytes to release ROS (esp. long fibers)
2. Fibers interfere physically with mitosis
— in vitro studies (high doses!), phagocytosis by target cells, interference
of long fibers with mitotic spindle and chromosome migration
3. Fibers stimulate proliferation of target cells
— compensatory cell proliferation
— stimulation of intracellular signal transduction pathways
— direct mitogenesis (proto-oncogene expression)
— induction of growth factors and growth factor receptor expression
4. Fibers provoke chronic inflammation and release of ROS, cytokines, and growth factors
— tissue injury, cell proliferation, inflammatory cells (neutrophils)
— link between sustained inflammation, fibrosis, cancer?
5. Fibers act as co-carcinogens or carriers of chemical carcinogens to target cells
— synergism between cigarette smoke and asbestos exposure
— interaction between fibrosis and non-fibrous dusts
Dr. Wallace’s Post-Meeting Comments
Health Effects of Asbestos and Synthetic Vitreous Fibers:
The Influence of Fiber Length
I participated in the Agency for Toxic Substances and Disease Registry (ATSDR) expert panel on “Health Effects of Asbestos and Synthetic Vitreous Fibers: The Influence of Fiber Length”, held in New York City on October 29-30, 2002. I limited my comments to one of the topics which ATSDR requested that the panel consider: “Topic #2: Health Effects of Asbestos and Vitreous Fibers less than 5 micrometers in length.” My research background has involved some studies of the surface properties and associated toxicities of respirable silica and silicate particulate dusts, which may have some indirect relevance to one of the questions asked of the panel under Topic #2, specifically: “Do the mechanisms of action of other materials (e.g., larger asbestos fibers, silicates, mineral dusts, amorphous silica) with potentially similar compositions aid in understanding small-fiber mechanisms of action?”
Dr. Ralph Zumwalde of NIOSH, who has an extensive background in the epidemiology of fiber-associated diseases, attended the proceedings as an observer and contributed information and recommendations concerning the availability of data and the analyses of epidemiology studies of occupational exposures to fibers.
In this review and revision of my comments on the panel, I also comment on the question: “Is there indirect evidence for less-than-5 micron fiber induced adverse health effects?” because of its association with the question of mechanism and because of some reports of inverse correlations of fiber length with fibrosis seen in asbestos workers’ lungs.
As discussed in the following review, my evaluation of information presented and commentary made by and to the panel is that there is a need for focused and short-term research on short fiber hazard; and that there are new opportunities for the design of that research.
Question: Do the mechanisms of action of other materials (e.g., larger asbestos fibers, silicates, mineral dusts, amorphous silica) with potentially similar compositions aid in understanding small-fiber mechanisms of action?A. Some lessons from non-fibrous particulate studies
1. Non-fibrous crystalline silica is cytotoxic, fibrogenic and carcinogenic.
Respirable crystalline silica particles, which are non-fibrous by any definition, are strongly pathogenic for fibrotic lung disease, and IARC, the US EPA, and others have recently evaluated quartz and cristobalite, two crystalline silica polymorphs, to be carcinogenic (1).
Exposure to these crystalline silica dusts can directly damage cells. Research suggests that consequent to this damage, there can be intrarcellular generation of reactive oxygen species and a cascade of events similar to the those evoked by asbestos fiber (2,3). As depicted by Dr. Mossman and others, that sequence may lead to the synthesis and release of TNF-alpha or other cytokines which stimulate near-by fibroblasts to proliferate and to up-regulate their synthesis and secretion of procollagen into the extracellular space of the pulmonary interstitium. There the procollagen matures into one or several forms of collagen fibers causing simple or progressive lung fibrosis.
The initial damage by quartz dust and by cristobalite dust to cells in vitro has been shown to be associated with the presence of silanols, hydroxyl groups on the crystalline silica surface. Bolasitis et al. (4,5) showed that calcining, e.g., heating, quartz resulted in the loss of surface silanols and a parallel loss of direct membranolytic cell damaging activity. As the dust aged in normal humidity air, the silanols re-formed on the surface over a period of days, and toxicity was restored parallel to that restoration. Saffiotti et al. (6) observed similar behavior with cristobalite. In some circumstances, e.g., sand-blasting occupational exposures, highly reactive free radical species are formed on the freshly broken crystalline silica surface; these exhibit heightened toxicity to cells in vitro in the absence of materials which can react to quench that activity, and may provide a additional mechanism of heightened toxicity (7).
2. Mineral-specific fibrogenicity: Short-term in vitro bioassays for mineral particles do not work
Some silicate dusts are cytotoxic in vitro but are not strongly pathogenic in vivo. Clays, layered alumino-silicates, are not associated with strong fibrogenic activity in human workplace exposures or in animal model exposure studies (8). In particular, respirable-sized kaolin clay dust, perhaps the structurally simplest alumino-silicate clay, is comparable to respirable-sized quartz dust for in vitro cytotoxicity (9) as measured by short term assays of cell damage, e.g., membranolysis, cytosolic or lysosomal enzyme release, or dye-exclusion measures of cell viability. Therefore, direct short-term in vitro cellular assays do not distinguish the distinct in vivo fibrogenic potentials of quartz versus kaolin clay dusts. Because of this, the general prevalence of clays in many mixed dust exposures prevents the use of short-term in vitro cytotoxicity systems to predict dust hazard.
3. The first events in particle or fiber interaction with the deep lung surface:
An important but generally ignored component for physiologically-representative in vitro bioassays:
a. The environmental interface of the deep lung is surfactant-coated
Particles or fibers depositing in the deep lung respiratory bronchioles or pulmonary alveoli will first contact the aqueous “hypophase” lining on the terminal airway and airsac surfaces. This thin layer is coated at the air-liquid interface with surfactant which acts to reduce the surface tension and physically stabilize the airspaces (10). The hypophase layer is also rich with micellar dispersion of surfactant. The surfactant is comprised principally of lipids and lipoproteins. The major constituents are phospholipids: diacyl phosphatidylcholines. Dipalmitoyl phosphatidyl choline (DPPC) dispersed in physiological saline provides perhaps the simplest model of lung surfactant, representing the major surfactant constituent and generally reproducing the surface tension-lowering characteristics of full lung surfactant.
b. toxic particles adsorb surfactant and are promptly neutralized
Both quartz and kaolin clay dust particles promptly adsorb DPPC surfactant from dispersion in physiological saline; this immediately coats the particle surfaces and prophylactically extinguishes their short-term cytotoxicity (12). The amounts of surfactant in the alveolar hypophase compared to the surface areas of respirable mineral dusts and their adsorption isotherms for DPPC suggest that there is adequate surfactant in the lung to coat and neutralize depositing particles even in most high dust exposures (13).
c. Restoration of particle toxicity and a possible basis for mineral-specific fibrogenicity
Subsequent to the suppression by pulmonary surfactant of otherwise prompt cytotoxic activity, the surfactant-coated particles can be phagocytized by macrophages and subjected to phagolysosomal enzymatic digestion (14). Cell-free experiments have correlated the digestive removal of DPPC from quartz and kaolin particle surfaces by phospholipase A2 enzyme with the restoration of membranolytic activity. In cell-free tests using pH -neutral acting phospholipase A2 and in limited in vitro/in vivo tests, quartz is stripped of surfactant significantly more rapidly that kaolin(15). Cellular in vitro studies have found that macrophage-like cells in vitro digest quartz- and kaolin-adsorbed DPPC at comparable rates over a period of about 7 to 10 days with initial partial restoration starting at 3 to 5 days (16). It has not been demonstrated that this de-toxification/re-toxification process is the mechanism distinguishing quartz and alumino-silicate expression of toxicity in vivo.
4. Site of particulate-induced fibrogenic activity
Churg et al. (17) briefly discuss the principal site of asbestos activity, noting the alveolar macrophage is commonly regarded as the crucial effector cell. This is the background assumption also for most experiments on the cytotoxic and fibrosis-associated activity of crystalline silica dusts. However, Adamson, referenced by Churg et al. in a different context, has published a suite of studies which make a case that it is interactions of silica particles with interstitial cells which control the stimulation of exacerbated collagen synthesis by pulmonary fibroblasts, and that the macrophage is responsible for only an inflammatory response evoking neutrophil influx to the alveolus but not tied to explicit fibrosis(18). While the mechanism of initial cell damage or stimulation may differ between silica or silicates and fibers, e.g., ROS from a “frustrated” phagocytosis mechanism for asbestos and surface silanol hydroxyl membranolysis by quartz or clay, a parallel analysis to Adamson’s silica study findings might be considered in researching the site of asbestos action for fibrosis.
5. Possible interferences in short-term bioassays
Oberdörster and others (19) have found that the conventional protocol for extended-term in vitro cellular assays may inadvertently cause a non-physiologic surface conditioning of mineral particles which significantly affects assay results. The use of fetal bovine serum can confer a prophylaxis on silica and perhaps on kaolin (20), probably due to the mineral surface adsorption of lipo-proteins from the FBS. That may not represent a physiological situation in the intact lung in vivo and may interfere with attempts to model the condition of particle surfaces upon deposition in the lung and resultant effects on their expression of toxicity in vivo. For purposes of in vitro investigation of fiber or particle toxicity, this interference might be circumvented, e.g., by excluding serum from the medium during a short-term period for particle or fiber challenge.
6. Environmental conditioning of particle surfaces can affect their in vivo pathogenic activity
Even animal model in vivo tests can fail to be predictive in the case of a cytotoxic and fibrogenic mineral in mixed composition dusts, e.g., quartz particles in workplace dusts: conventional mineralogical and cytotoxicity assays may not correlate with short-or intermediate-term in vivo fibrogenic response. Alumino-silicate surface contamination of quartz particle surfaces can delay for months or perhaps years the expression of fibrogenic activity. Aluminosilicate or other mineral occlusion of the underlying host particle can alter the expression of toxicity in vivo during the bio-persistence of the surface contamination. This has been seen worldwide in anomalies in the fibrogenicity of coal mine dust exposures (21). This was clearly demonstrated by LeBouffant et al. (22) by in vitro and in vivo studies of the fibrogenicity of silica in coal mine dusts and in natural lightly contaminated sands. More recently, new spectroscopic surface analysis methods have demonstrated natural clay occlusion of quartz dusts from some workplace where epidemiology studies had detailed anomalies in disease risk correlation with conventional measures of dust exposure (23).
B. Fibrous mineral and crystalline silica particle differences and similarities
1. Mechanisms of toxicity for fibrous and non-fibrous materials
a. Conventional assays do not clarify the bases of asbestos or silica particle toxicity
Churg et al. (17) review highlights and caveats to the general models of asbestos activity. Some fibers can evoke the responses from ROS generation through the cascade to increased expression of TNF-alpha, but have not been shown to induce fibrosis. And asbestos produces fibrosis in some systems without increasing TNF-alpha expression. Chrysotile contains little iron but is fibrogenic, albeit not a potent as amphibole. Churg et al. suggest a comparison of asbestos and silica-induced fibrosis data. Their paper compares the generation of ROS, RNS, and activation of NF-kB and AP-1, and increased production of TNF-alpha and other factors and find the dusts to be indistinguishable. In the face of this, asbestosis and silicosis differ in histopathological appearance: asbestosis is a diffuse fibrosis and silicosis is in localized nodules. Their conclusion is that the tabulated responses fail to explain comprehensively how asbestosis or silicosis develop.
b. Surfactant does not fully suppress all asbestos fiber in vitro cytotoxicity
Asbsetos fiber as well as particulate silicate can adsorb the DPPC and components of pulmonary surfactant (24). We have briefly researched the effect of surfactant adsorption on chrysotile in vitro genotoxicity, using an assay for micronucleus induction in cultured Chinese hamster lung cells (V79 cells) (25): in our test of two chrysotile asbestos fiber samples, pre-treatment with DPPC in physiological saline surrogate lung surfactant did not fully suppress a short-term toxic activity to cells in vitro. NIEHS intermediate length chrysotile asbestos fiber (average 101 micrometer length, 65% > 10 micron) and NIEHS short chrysotile asbestos fiber (average 11.6 micron, 98% < 10 micron) were tested for micronucleus induction in V79 macrophage-derived cells for 72 hour challenge +/- DPPC surfactant pre-treatment of the fibers. For the longer fiber sample, DPPC did not significantly affect the activity, a numerical reduction of about 20% in the activity was observed but was not statistically significant. However, DPPC treatment reduced the shorter-length fiber sample activity significantly, to about half that of the untreated shorter fiber sample. Similar effects were seen for multi-nuclei induction and for dye-exclusion viability measure for cell toxicity. No activity was seen for either sample in a sister chromatid exchange assay.
c. A surface modification which did not affect long asbestos fiber toxicity in vitro
We also attempted to see if a significant surface modification of chrysotile without a significant modification of fiber size would affect in vitro genotoxic activity (26). The NIEHS intermediate length chrysotile asbestos fiber used above was mildly acid leached to remove near-surface magnesium, but to retain fiber length. The treatment resulted in a 20% reduction in fiber length in each of three length categories: <3 micron, 3-10 micron, > 10 micron. Spectroscopic surface analysis and zeta-potential measurements showed significant reduction in surface-associated magnesium and in its influence on surface chemistry. However there was no significant change in measured activity for micronucleus induction between the treated and non-treated fibers.
d. Is there more than one mechanism of fiber cytotoxicity? Do short fibers also act as particles?
One interpretation of these two experiments is this: at least two mechanisms are involved in the initial damage or interaction of fibrous particles with the lung: a component which is at least transiently suppressed by surfactant conditioning which significantly contributes to shorter fiber activity, and a component which is not suppressed by surfactant conditioning, and which is not affected by one significant modification of surface composition and chemistry, and which is the principal mechanism for longer fibers. That is, a model which suggests itself is the combination of the frequently discussed “frustrated phagocytosis” mechanism for longer fibers, e.g., those which are too long to be fully phagocytized and internalized by the cell target, and a surface property-mediated toxicity mechanism for internalized particles or short fibers, i.e., fibers which are internalized and subjected to conventional phagolysosomal processes.
One possible consequence of “frustrated phagocytosis” of longer fibers is that the partially invaginated fiber stimulates the cell to release superoxide in a manner related to the respiratory burst upon normal phagocytosis, or that superoxide is produced by the cell in response to an autolytic effect of enzymes or other lysosomal or cytosolic agents released into the annular invagination of the fiber. The superoxide is then in close approximation with reactive iron species on the fiber surface in or extending beyond the partially invaginated fiber to create hydroxyl radical for strongly toxic effects at the cell or neighboring cells. The paper by M Ohyama et al. (27) provided to the panel presents a difficult argument against frustrated phagocytosis: The study used luciginen-dependent chemiluminescence (CL) induced in vitro over a short (2 hour) period, and found a strong correlation of response indicative of superoxide release with fiber length 6 to 20 um. All samples except wollastonite induced CL response in a dose-dependent manner. Superoxide release was non-specific for compositional type of fiber. The four fibers with lengths below 7 um were only weakly active. Longer fiber activity correlated with length.
Research on the surfactant suppression and subsequent lysosomal enzymatic restoration of mineral particle cytotoxicity within a cell, suggests that short fibers which are fully taken into the cell in a phagosome may express, in part, a cytotoxicity within the cell after removal of adsorbed prophylactic surfactant. That is, some part of short fiber toxicity may be related to the mineral surface-specific mechanism of non-fibrous particulate toxicity.
Those do not exhaust the possible mechanisms for long or short fiber damage to cells. Asbestos fiber penetrating the cell or cell nucleus may exercise modes of direct genetic or epigenetic damage. In our above study of surfactant effects on chrysotile genotoxicity in vitro, a limited investigation using immunofluorescent kinetochore staining indicated that both clastogenic and aneuploidogenic effects were associated in similar proportion with the observed micronucleus induction. That is, fibers may directly or indirectly interact with the spindle mechanism involved in chromosomal separation during cell division. During mitosis, the nuclear membrane disintegrates, possibly providing intracellular fibers access to the genetic material or kinetochores and spindle apparatus.
2. Intracellular response to fiber challenge
a. Long fiber challenge
Whatever the mechanisms of direct fiber damage or stimulation of the cell surface, some components of the consequent intracellular response have been well-defined. Mossman and others have detailed the cascade of events following fiber challenge to pulmonary macrophages or perhaps to other cells. A recent review (3) explicates the central dogma that damage to or stimulation of the cell by fibers is followed by an increase in intracellular reactive oxygen species which trigger a cascade of transcription factor activation leading to the up-regulated production and release of TNF-alpha or other cytokines. This also was recently the subject of a NIOSH study by Cheng et al. (28) in which crocidolite with a median fiber length of 11.5 um challenged lavaged rat AM in FBS-containing medium for 1 to 24 h.. Crocidolite caused parallel increases in TNF-alpha production and NF-kB activation.in a dose-dependent manner. A titanium oxide control dust had no stimulatory effect on TNF-a secretion. The report by V Kinnula which was provided to the panel (29) reviews the possible roles of reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated by asbestos fiber in cell-free and cellular and tissue systems. A primary step in response to asbestos “long” fiber challenge of cells is agreed to be superoxide anion release in cells which have attempted to phagocytize fibers. This superoxide can further be dismutated to hydrogen peroxide, which can generate hydroxyl radical, catalyzed by iron via the Fenton reaction. That hydroxyl radical is extremely toxic and reactive, but therefore short-lived. There is some contention that fibers stimulate the release of ROS from inflammatory cells and not target cells. However, asbestos fiber can generate ROS spontaneously in cell-free systems. This fiber-prompted production and release of TNF-alpha can stimulate nearby pulmonary fibroblasts to proliferate and increase pro-collagen synthesis, which is released extra-cellularly to mature into collagen scarring.
b. Intracellular response to challenge by well-classified shorter fibers and particles
Dr. Baron of NIOSH has been developing a fiber size classifier (separator) which can permit in vitro or perhaps limited in vivo experiments with sets of fibers of fairly well-defined length (30). A dielectrophoretic classifier can separate fibers from an airstream producing about 1 mg/day of a size cut. These classes of JM-100 glass fibers were recently produced for in vitro toxicology study:
cut 1: Length = 32.7 micrometer +/- 23.5 SD; Width = 0.75 micrometer +/- 0.50
micrometer
cut 2: L = 16.7 u +/- 10.6 u; W = 0.49 u +/- 0.27 u
cut 3: L = 6.5 u +/- 2.7 u; W = 0.44 u +/- 0.22 u
cut 4: L = 4.3 u +/- 1.0 u; W = 0.40 +/- 0.15 u
cut 5: L = 3.0 u +/- 1.0 u; W = 0.35 u +/- 0.14 u
In recent NIOSH studies by Dr. Castranova and colleagues, these samples were used in a comparison of “long” and “short” fiber cytotoxicity and of induction of the cytokine cascade in vitro: Blake et al. (31) used 18 hour challenge of rat alveolar macrophages in vitro and the lactate dehydrogenase (LDH) release assay, the 17 micrometer sample expressed about 2 X the activity of the shorter samples (and also 2X the activity of the 33 micrometer longer sample) on a mass basis. However, all samples were active well above control levels. The 7 micrometer fiber set had about 8 X more fibers per gram than the 17 micrometer set, or about 3 X the linear surface area. Thus, the 17 micrometer long fibers were on the order of 6 or 7 X more cytotoxic than the shorter 7 micrometer fibers on a linear surface basis. Similar effects were seen with an assay using chemiluminescent response to zymosan challenge. And multiple macrophages were seen attached along the length of the long fibers, suggesting “frustrated” or incomplete phagocytosis was occurring for longer fibers.
J Ye et al.(32) challenged a mouse macrophage cell line with the 7 and with the 17 um glass fiber cuts, for 3, 6, and 16 h. Glass fibers stimulated TNF-alpha production, activation of TNF-alpha gene promoter activity, and activation of DNA binding activity of nuclear factor (NF)-kB. Reactive oxygen species (ROS) were involved in the activation and production. Dose was set at 5 fibers per cell; by that metric the longer fibers were more potent than short fibers by a factor of about 3. However, on a basis of length of fiber exposed to the cell or surface area, the activities were about equal for the long and short fibers. As seen in photomicrographs, short fibers but not long fibers were effectively engulfed by macrophages. In a subsequent study by Ye et al. (33) it was found that the long fibers were more potent than short fibers at the same dose of 5 fibers/cell in activating MAP kinases which activate transcription factor c-Jun which acts on the TNF-a gene promoter through the cyclic AMP response element and the AP-1 binding site. Again, the activities were comparable for long and short fibers on the basis of exposed fiber length or surface area.
| Question: | Is there indirect evidence for less-than-5 micron fiber induced adverse health effects? |
A. Human studies
1. Churg et al. (34) found the grade of interstitial fibrosis asbestosis in the lungs of a group of chrysotile miners and millers to be directly proportional to tremolite or chrysotile fiber concentrations, but inversely proportional to mean fiber length and length-related parameters. Churg et al., (35) graded fibrosis in the lungs of some shipyard and insulation workers, finding fibrosis grade to be strongly positively correlated with amosite concentration and negatively correlated with mean fiber size parameters including fiber length; they suggested “...these observations again raise the possibility that short fibers may be more important than is commonly believed in the genesis of fibrosis in man.” In a study of chrysotile miners and millers, Churg, et al., found pleural plaques were strongly associated with mean tremolite fiber aspect ratio, but no differences in mean fiber size, including length, were seen for any other disease studied (mesothelioma, airway fibrosis, asbestosis, or carcinoma) (36). One member brought to the panel’s attention a recent publication (37) analyzing fibers in lung tissue from two groups of former chrysotile miners and millers: the study concluded that “...fiber dimension does not seem to be a factor that accounts for the difference in incidence of respiratory disease between the two groups”. It has been generally speculated that shorter fibers in lung tissue may be the residue of fibers which were longer when deposited, and disease initiation was due to the originally long fibers, which were subjected to subsequent in vivo dissolution or degradation into the observed short fibers (17). This appears to be one plausible explanation of the inverse correlations reported between fibrosis and fiber length in human lungs. But this does not limit the research opportunity or imperative, provided by the seemingly anomalous or “counter-intuitive” results, to address possible short fiber-associated disease mechanisms.
2. A possible “short fiber” exposure cohort. Dr. Zumwalde of NIOSH suggested to the panel that a past NIOSH study of 2,302 workers at an attapulgite mining and milling facility (38) may have involved exposures, in part, to short mineral fibers. A significant deficit of mortality (SMR = 43, 90% CI 23-76) from nonmalignant respiratory disease (NMRD) was observed for the cohort; but a statistically significant excess of mortality from lung cancer was observed among whites (SMR = 193, 90% CI 121-293), but a deficit occurred among nonwhites (SMR = 53, 90% CI 21-112). This may present an opportunity for review and re-analysis and a source for collection of materials for study. NIOSH also is re-analyzing archived materials available from a past study of asbestos workers in South Carolina.
Question:Are short fibers pathogenic? What should we do?
1. Review of in vitro toxicology
For non-fibrous particles:
- Non-fibrous mineral particles can be cytotoxic, fibrogenic, and carcinogenic.
- That pathogenicity is mineral-specific.
- Surface characteristics may delay expression of that pathogenic activity in
vivo.
- That pathogenicity is not necessarily reflected in short-term in vitro cytotoxicity
assays.
- The first interaction of particles depositing in the deep lung, namely, adsorption
of the lung lining surfactant, strongly affects mineral particle prompt toxicity.
- The bio-persistence of that surfactant prophylaxis may be a critical factor
in the timing and severity of mineral particle expression of toxicity.
- After expression of the primary toxic event in particle challenge to cells,
the intracellular response may be much similar to the cascade induced by asbestos
or fiber challenge: leading to the induction of pathogenic, e.g., fibrogenic
activity by nearby cells.
For fibrous particles:
- Many studies have found an association of pulmonary fibrosis, cancer, and
mesothelioma with occupational exposures to long fibers, e.g., fibers with length
greater than the dimensions of the target cells.
- Long fibers clearly are cytotoxic in vitro.
- Long fiber cytotoxicity and the initiation of pathogenic processes are generally
considered to be resultant from a “frustrated phagocytosis” mechanism.
- Some studies of fiber burden and disease in tissue from asbestos workers have
shown an inverse correlation of disease with fiber length.
- Those disease-correlated shorter fibers appear in some of the cases to be
mineral specific, e.g., associated with contaminant amphibole more than with
seprentine asbestos.
- Limited in vitro study data suggest that shorter fibers may have a component
of cytotoxicity which is surface associated, perhaps independent of a “frustrated
phagocytosis” mechanism involved in long fiber toxicity.
- In short term in vitro assays, well-controlled for fiber length, shorter glass
fibers can cause intracellular events comparable on a fiber or surface basis
to those associated with longer fiber challenge and with asbestos fiber challenge.
2. Interpretability of short-term in vitro assays
The ability to interpret many in vitro experiments on particle of fiber toxicity
is complicated by the lack of modeling of initial conditioning of particles
in the lung and the time course of expression of toxicity in vivo. Surfactant
adsorption in the lung can dramatically alter the short-term in vitro toxicities
of mineral particles, and may determine if toxicity is expressed in times long
or short compared to clearance.
This surfactant effect and associated delay in toxicity expression does not appear to be a factor for long fiber asbestos expression of in vitro toxicity. Whether this is a factor for short fibers is unknown. That is, if short fibers have a component of toxicity independent of a “frustrated phagocytosis” mechanism but dependent on a surface-property mechanism then such conditioning and time delays in expression of toxicity could be critical in the design of experiments for the detection and analysis of short fiber toxicity by in vitro or short-term in vivo assay.
3. Research opportunities
a. The ability to collect milligram quantities of well-classified (sized) small fibers presents the opportunity to do carefully size-controlled in vitro studies and possibly some (more limited) in vivo studies, e.g., by nose-only inhalation or tracheal instillation.
b. Epidemiology study results suggest types of fibers which should be compared and contrasted in such experiments, e.g., short tremolite vs. short chrysotile.
c. A review of epidemiological studies of attapulgite or other short fiber exposures may provide an identification of other short fiber asbestos and non-asbestos materials for toxicological study for which human disease epidemiology information is available for comparison.
d. Preceding the initiation of new toxicology studies, a review of past in vitro studies might identify the controls for surface conditioning of the test fibers: were effects of lung conditioning modeled, or were non-physiologic effects of medium adsorbates possible in past studies?
e. So-designed in vitro toxicology studies of classified short fibrous materials
which have known positive or negative correlations with pathology could be attempted
to determine if there is a short fiber toxicity with a reasonable potential
to initiate disease in vivo, and if that potential is
dependent on fiber mineral type or surface property or morphology.
f. A similar review of short-term in vivo studies might help the design of methods of challenge and time course for tests of materials selected from the in vitro study results.
g. Results of the in vitro and in vivo studies would suggest if useful application could be made to dusts of current concern, e.g., Libby vermiculite or World Trade Center disaster-associated dusts.
References
1. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Vol. 68: “Silica, some silicates, coal dust, and para-aramid fibrils”. ISBN 92 832 1268 1, 1997.
2. Driscoll, K. The role of interleukin-1 and tumor necrosis factor-a in the lung’s response to silica. In: Silica and Silica-Induced Lung Diseases, V Castranova, V Vallyathan, and W Wallace, eds., Boca Raton, FL., pp163-184, 1996.
3. C Manning, V Vallyathan, and B Mossman: “Diseases caused by asbestos: mechanisms of injury and disease development” International Immunopharmacology 2:191-200, 2002.
4. Razzaboni BL, Bolsaitis P, Wallace WE, Keane MJ. “Effect of thermal treatment on the surface characteristics and hemolytic activity of respirable size silica particles. Proc. Of the VIIth International Pneumoconioses Conference, Pittsburgh, PA, 1988. DHHS (NIOSH) Publ. No. 90-108 Part 1, 215-230, 1990.
5. Pandurangi RS, Seehar MS, Razzaboni BL, Bolsaitis. “Surface and bulk infrared modes of crystalline and amorphous silica particles: a study of the relation of surface structure to cytotoxicity of respirable silica. Environ. Health Perspect. 86:317-336, 1990.
6. Fubini B, Zanetti G, Altilia J, Tiozzo R, Lison D, Saffiotti U. “Relationship between surface properties and cellular responses to crystalline silica: studies with heat-treated cristobalite”. Chem res Toxicol 12(8): 737-745, 1999.
7. Castranova V, Dalal NS, Vallyathan V. “Role of surface free radicals in the pathogenicity of silica”. In: Silica and Silica-Induced Lung Diseases, V Castranova, V Vallyathan, and W Wallace, eds., Boca Raton, FL., pp91-105, 1996.
8. Gamble J. “Silicate pneumoconioses” in “Occupational Respiratory Diseases”, ed. JA Merchant.pp. 243-284 . DHHS, (NIOSH) Publication No. 86-102., 1986.
9. Vallyathan, V, D.Schwegler, M Reasor, L Stettler, FHY Green (1988). Comparison of in vitro cytotoxicity and relative pathogenicity of mineral dusts. Ann. Occ. Hyg. 32, 279-289.
10. King RJ, Clements JA. “Surface active materials from dog lung.I. Method of isolation.” Am J Physiol. 223:707-726, 1972.
11. Wallace, WE, V. Vallyathan, MJ Keane, V. Robinson (1985). In vitro biologic toxicity of native and surface-modified quartz and kaolin. J. Tox. Env. Health 16:415-424.
13. Wallace WE, Headley LC, Weber KC. “Dipalmitoyl lecithin surfactant adsorption by kaolin dust in vitro.” J Colloidal Interface Sci 51:535, 1975.
14. Allison AC. “Lysosomes and the toxicity of particulate pollutants”. Arch. Intern. Med. 128: 131, 1971.
15. Wallace, W, M Keane, P Mike, C Hill, V Vallyathan, and E Regad (1992). Contrasting respirable quartz and kaolin retention of lecithin surfactant and expression of membranolytic activity following phospholipase A2 digestion. J Tox. Environ. Health 37:391-409.
16. Hill, C, W Wallace, M Keane, and P Mike (1995). The enzymatic removal of a surfactant coating from quartz and kaolin by P388D1 cells. Cell Biol. Tox. 11:119-128.
17. Churg et al. “Pathogenesis of fibrosis produced by asbestos and man-made mineral fibers: what makes a fiber fibrogenic?”, Inhalation Toxicology 12(S3):15-26, 2000,
18. Bowden DH; Hedgecock C; Adamson IY(1989). Silica-induced pulmonary fibrosis involves the reaction of particles with interstitial rather than alveolar macrophages. J Patho1 58:73-80.
19. Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. “Surum binds serum proteins resulting in a shift of the dose-response for silica-induced chemokine expression in an alveolar type II cell line.” Toxicol. Appl. Pharmacol. 161: 111-122,1999.
20. Gao N, Keane MJ, Ong T, Ye J, Miller WE, Wallace WE. “Effects of phospholipid surfactant on apoptosis induction by respirable quartz and kaolin in NR8383 rat pulmonary macrophages”. Toxicol. Appl. Pharmacol. 175: 217-225, 2001.
21. Robock K, Klosterkotter W. “Investigations into the specific toxicity of different SiO2 and silica dusts. Staub Reinhart Luft 33:3360-3363, 1973.
22. LeBouffant L, Daniel H, Martin J,C, Bruyere S. “Effect of impurities and associated minerals on quartz toxicity. Ann. Occup. Htg. 26: 625-634, 1982.
23. Harrison J, Brower PS, Attfield MD, Doak CB, Keane MJ, Grayson RL, Wallace WE. “Surface composition of respirable silica particles in a set of US anthracite and bituminous coal mine dusts”. J Aerosol Sci. 28:689-696, 1997.
24. Jaurand MC, Reiner A, Bignon J. “The adsorption of phospholipids and red blood cell membranes on chrysotile fibers”. In “In Vitro Effects of Mineral Dusts”. Ed. RC Brown. Academic Press, London1980: 125-130.
25. Liu J “In vitro genotoxicity studies of chrysotile asbestos fibers dispersed in simulated pulmonary surfactant” Mutation Res 320: 253-259, 1994.
26. Keane al. “A study of the effect of chrysotile fiber surface composition on genotoxicity in vitro”. J Tox & Environm Hlth:57:529-541, 1999.
27. M Ohyama, T Otake, and K Morinaga, “Effect of size of man-made and natural mineral fibers on chemiluminescent response in human monocyte-derived macrophages.” Environ Hlth Perspec 109:1033-1039, 2001.
28. Cheng et al. “Role of transcription factro NF-kB in asbestos-induced TNF-alpha response from Macrophages” Expt. And Mol Pathology 66:201-210, 1999.
29. V Kinnula which was provided to the panel, “Oxidant and antioxidant mechanisms of lung disease caused by asbestos fibers” European Respiratory Journal 14(3):706-716, 1999
30. Baron et al. “Length separation of fibers”. Aerosol Sci. Tech. 21:179-192, 1994.
31. Blake et al. “Effects of fiber length on glass microfiber toxicity” J Toxicol Environ Hlth 54: 243-259, 1998.
32. J Ye et al. “Critical role of glass fiber length in TNF-alpha production and transcription factor activation in macrophages.” Am J Physiol276 (Lung Cell Mol Physiol 20):L426-L434, 1999.
33. Ye et al. “Activation of mitogen-activated protein kinase p38 and extracellular signal-regulated kinase is involved in glass fiber-induced tumor necrosis factor-alpha production in macrophages” J Biological Chem 276:5360-5367,2001.
34. Churg et al. ”Mineralogical correlates of fibrosis in chrysotile miners and millers”. Am Rev Resp Dis 139: 891-896, 1990.
35. Churg et al. “Mineralogical parameters related to amosite asbestos-induced fibrosis in humans” Am Rev Resp Dis 142: 1331-1336, 1990.
36. Churg et al. “Fiber burdens and patterns of asbestos-related diseases in
workers with
heavy mixed amosite and chrysotile exposures”, Am J Resp Crit Care Med 150:
663-669, 1994.
37. Nayebzadeh A, Dufrense A, Case B, et al. “Lung mineral fibers of former miners and millers from Thetford-Mines and asbestos regions: a comparative study of fiber concentration and dimension.” Arch Environ Health 56(1):65-76, 2001.
38. Waxweiler RJ, Zumwalde RD, Ness GO, Brown DP. “A retrospective cohort mortality study of males mining and milling attapulgite clay”. Am J Ind Med 13(3): 305-315, 1988.


