Chapter 2: Landfill Gas Basics
This chapter provides basic information about landfill gas—what it is composed of, how it is produced, and the conditions that affect its production. It also provides information about how landfill gas moves and travels away from the landfill site. Finally, the chapter presents an overview of the types of landfills that might be present in your community and the regulatory requirements that apply to each.
What is landfill gas composed of?
Landfill gas is composed of a mixture of hundreds of different gases. By volume, landfill gas typically contains 45% to 60% methane and 40% to 60% carbon dioxide. Landfill gas also includes small amounts of nitrogen, oxygen, ammonia, sulfides, hydrogen, carbon monoxide, and nonmethane organic compounds (NMOCs) such as trichloroethylene, benzene, and vinyl chloride. Table 2-1 lists "typical" landfill gases, their percent by volume, and their characteristics.
How is landfill gas produced?
Three processes—bacterial decomposition, volatilization, and chemical reactions—form landfill gas.
- Bacterial decomposition. Most landfill
gas is produced by bacterial decomposition, which occurs when
organic waste is broken down by bacteria naturally present in
the waste and in the soil used to cover the landfill. Organic
wastes include food, garden waste, street sweepings, textiles,
and wood and paper products. Bacteria decompose organic waste
in four phases, and the composition of the gas changes during
each phase. The box below provides detailed
information about the four phases of bacterial decomposition
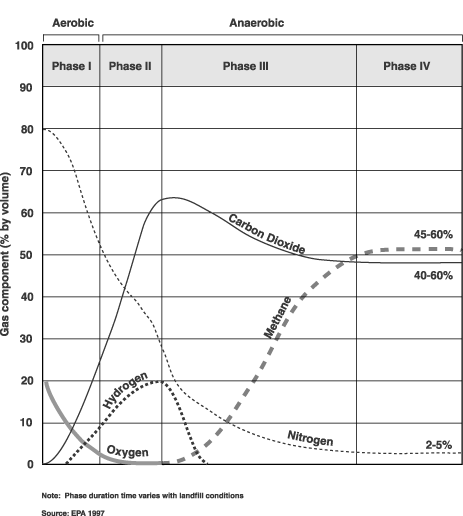
and the gases produced during each phase. Figure
2-1 shows gas production at each of the four stages.
- Volatilization. Landfill gases can be created
when certain wastes, particularly organic compounds, change
from a liquid or a solid into a vapor. This process is known
as volatilization. NMOCs in landfill gas may be the result of
volatilization of certain chemicals disposed of in the landfill.
- Chemical reactions. Landfill gas, including NMOCs, can be created by the reactions of certain chemicals present in waste. For example, if chlorine bleach and ammonia come in contact with each other within the landfill, a harmful gas is produced.
|
Table 2-1: Typical Landfill Gas Components
|
||
|---|---|---|
| Component | Percent by Volume | Characteristics |
| methane | 45–60 | Methane is a naturally occurring gas. It is colorless andodorless. Landfills are the single largest source of U.S. man-made methane emissions |
| carbon dioxide | 40–60 | Carbon dioxide is naturally found at small concentrations in the atmosphere (0.03%). It is colorless, odorless, and slightly acidic. |
| nitrogen | 2–5 | Nitrogen comprises approximately 79% of the atmosphere. It is odorless, tasteless, and colorless. |
| oxygen | 0.1–1 | Oxygen comprises approximately 21% of the atmosphere. It is odorless, tasteless, and colorless. |
| ammonia | 0.1–1 | Ammonia is a colorless gas with a pungent odor. |
| NMOCs (non-methane organic compounds) |
0.01–0.6 | NMOCs are organic compounds (i.e., compounds that contain carbon). (Methane is an organic compound but is not considered an NMOC.) NMOCs may occur naturally or be formed by synthetic chemical processes. NMOCs most commonly found in landfills include acrylonitrile, benzene, 1,1-dichloroethane, 1,2-cis dichloroethylene, dichloromethane, carbonyl sulfide, ethyl-benzene, hexane, methyl ethyl ketone, tetrachloroethylene, toluene, trichloroethylene, vinyl chloride, and xylenes. |
| sulfides | 0–1 | Sulfides (e.g., hydrogen sulfide, dimethyl sulfide, mercaptans) are naturally occurring gases that give the landfill gas mixture its rotten-egg smell. Sulfides can cause unpleasant odors even at very low concentrations. |
| hydrogen | 0–0.2 | Hydrogen is an odorless, colorless gas. |
| carbon monoxide | 0–0.2 | Carbon monoxide is an odorless, colorless gas. |
|
Source: Tchobanoglous, Theisen, and Vigil 1993; EPA
1995
|
||
| The Four Phases of Bacterial Decomposition of Landfill Waste |
|---|
Bacteria decompose landfill waste in four phases. The composition of the gas produced changes with each of the four phases of decomposition. Landfills often accept waste over a 20- to 30-year period, so waste in a landfill may be undergoing several phases of decomposition at once. This means that older waste in one area might be in a different phase of decomposition than more recently buried waste in another area. Phase I Phase II Phase III Phase IV
|
Figure 2-1: Production phases of typical landfill gas
What conditions affect landfill gas production?
The rate and volume of landfill gas produced at a specific site depend on the characteristics of the waste (e.g., composition and age of the refuse) and a number of environmental factors (e.g., the presence of oxygen in the landfill, moisture content, and temperature).
- Waste composition. The more organic waste
present in a landfill, the more landfill gas (e.g., carbon dioxide,
methane, nitrogen, and hydrogen sulfide) is produced by the
bacteria during decomposition. The more chemicals disposed of
in the landfill, the more likely NMOCs and other gases will
be produced either through volatilization or chemical reactions.
- Age of refuse. Generally, more recently
buried waste (i.e., waste buried less than 10 years) produces
more landfill gas through bacterial decomposition, volatilization,
and chemical reactions than does older waste (buried more than
10 years). Peak gas production usually occurs from 5 to 7 years
after the waste is buried.
- Presence of oxygen in the landfill. Methane
will be produced only when oxygen is no longer present in the
landfill.
- Moisture content. The presence of moisture
(unsaturated conditions) in a landfill increases gas production
because it encourages bacterial decomposition. Moisture may
also promote chemical reactions that produce gases.
- Temperature. As the landfill's temperature rises, bacterial activity increases, resulting in increased gas production. Increased temperature may also increase rates of volatilization and chemical reactions. The box on the following page provides more detailed information about how these variables affect the rate and volume of landfill gas production.
How does landfill gas move?
Once gases are produced under the landfill surface, they generally move away from the landfill. Gases tend to expand and fill the available space, so that they move, or "migrate," through the limited pore spaces within the refuse and soils covering of the landfill. The natural tendency of landfill gases that are lighter than air, such as methane, is to move upward, usually through the landfill surface. Upward movement of landfill gas can be inhibited by densely compacted waste or landfill cover material (e.g., by daily soil cover and caps). When upward movement is inhibited, the gas tends to migrate horizontally to other areas within the landfill or to areas outside the landfill, where it can resume its upward path. Basically, the gases follow the path of least resistance. Some gases, such as carbon dioxide, are denser than air and will collect in subsurface areas, such as utility corridors. Three main factors influence the migration of landfill gases: diffusion (concentration), pressure, and permeability
- Diffusion (concentration). Diffusion describes
a gas's natural tendency to reach a uni-form concentration in
a given space, whether it is a room or the earth's atmosphere.
Gases in a landfill move from areas of high gas concentrations
to areas with lower gas concentrations. Because gas concentrations
are generally higher in the landfill than in the surrounding
areas, landfill gases diffuse out of the landfill to the surrounding
areas with lower gas concentrations.
- Pressure. Gases accumulating in a landfill
create areas of high pressure in which gas movement is restricted
by compacted refuse or soil covers and areas of low pressure
in which gas movement is unrestricted. The variation in pressure
throughout the landfill results in gases moving from areas of
high pressure to areas of low pressure. Movement of gases from
areas of high pressure to areas of lower pressure is known as
convection. As more gases are generated, the pressure in the
landfill increases, usually causing sub-surface pressures in
the landfill to be higher than either the atmospheric pressure
or indoor air pressure. When pressure in the landfill is higher,
gases tend to move to ambient or indoor air.
- Permeability. Gases will also migrate according to where the pathways of least resistance occur. Permeability is a measure of how well gases and liquids flow through connected spaces or pores in refuse and soils. Dry, sandy soils are highly permeable (many connected pore spaces), while moist clay tends to be much less permeable (fewer connected pore spaces). Gases tend to move through areas of high permeability (e.g., areas of sand or gravel) rather than through areas of low permeability (e.g., areas of clay or silt). Landfill covers are often made of low-permeability soils, such as clay. Gases in a covered landfill, therefore, may be more likely to move horizontally than vertically.
|
Table 2-2: Factors Affecting Landfill Gas Production
|
|---|
|
Waste Composition. The more organic waste present in a landfill, the more landfill gas is produced by bacterial decomposition. Some types of organic waste contain nutrients, such as sodium, potassium, calcium, and magnesium, that help bacteria thrive. When these nutrients are present, landfill gas production increases. Alternatively, some wastes contain compounds that harm bacteria, causing less gas to be produced. For example, methane-producing bacteria can be inhibited when waste has high salt concentrations. Oxygen in the Landfill. Only when oxygen is used up will bacteria begin to produce methane. The more oxygen present in a landfill, the longer aerobic bacteria can decompose waste in Phase I. If waste is loosely buried or frequently disturbed, more oxygen is available, so that oxygen-dependent bacteria live longer and produce carbon dioxide and water for longer periods. If the waste is highly compacted, however, methane production will begin earlier as the aerobic bacteria are replaced by methane-producing anaerobic bacteria in Phase III. Methane gas starts to be produced by the anaerobic bacteria only when the oxygen in the landfill is used up by the aerobic bacteria; therefore, any oxygen remaining in the landfill will slow methane production. Barometric highs will tend to introduce atmospheric oxygen into surface soils in shallow portions of a landfill, possibly altering bacterial activity. In this scenario, waste in Phase IV, for example, might briefly revert to Phase I until all the oxygen is used up again. Moisture Content. The presence of a certain amount of water in a landfill increases gas production because moisture encourages bacterial growth and transports nutrients and bacteria to all areas within a landfill. A moisture content of 40% or higher, based on wet weight of waste, promotes maximum gas production (e.g., in a capped landfill). Waste compaction slows gas production because it increases the density of the landfill contents, decreasing the rate at which water can infiltrate the waste. The rate of gas production is higher if heavy rainfall and/or permeable landfill covers introduce additional water into a landfill. Temperature. Warm temperatures increase bacterial activity, which in turn increases the rate of landfill gas pro-duction. Colder temperatures inhibit bacterial activity. Typically, bacterial activity drops off dramatically below 50° Fahrenheit (F). Weather changes have a far greater effect on gas production in shallow landfills. This is because the bacteria are not as insulated against temperature changes as compared to deep landfills where a thick layer of soil covers the waste. A capped landfill usually maintains a stable temperature, maximizing gas production. Bacterial activity releases heat, stabilizing the temperature of a landfill between 77° F and 113° F, although temperatures up to 158° F have been noted. Temperature increases also promote volatilization and chemical reactions. As a general rule, emissions of NMOCs double with every 18° F increase in temperature. Age of Refuse. More recently buried waste will produce more gas than older waste. Landfills usually produce appreciable amounts of gas within 1 to 3 years. Peak gas production usually occurs 5 to 7 years after wastes are dumped. Almost all gas is produced within 20 years after waste is dumped; however, small quantities of gas may continue to be emitted from a landfill for 50 or more years. A low-methane yield scenario, however, estimates that slowly decomposing waste will produce methane after 5 years and continue emitting gas over a 40-year period. Different portions of the landfill might be in different phases of the decomposition process at the same time, depending on when the waste was originally placed in each area. The amount of organic material in the waste is an important factor in how long gas production lasts. |
|
Sources: Crawford and Smith 1985; DOE 1995;
EPA 1993.
|
Table of Contents Next Section
Contact Us:
- Agency for Toxic Substances and Disease Registry
4770 Buford Hwy NE
Atlanta, GA 30341-3717 USA - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348
Email CDC-INFO - New Hours of Operation
8am-8pm ET/Monday-Friday
Closed Holidays


