What Are Possible Health Effects from Lead Exposure?
This page refers to a blood lead level of 5 micrograms per deciliter (μg/dL) as the CDC’s blood lead reference value. As of October 28, 2021, CDC uses a blood lead reference value of 3.5 micrograms per deciliter to identify children with blood lead levels that are higher than most children’s levels. This new level is based on the U.S. population of children ages 1–5 years who represent the top 2.5% of children with the highest blood lead levels. For more information, refer to Blood Lead Reference Value.
Upon completion of this section, you will be able to
- Describe how lead affects adults and children,
- Describe what is the most sensitive organ system for lead exposure in children, and
- Specify that there is no identified threshold or safe level of lead in blood.
Blood lead concentrations have decreased dramatically in U.S. children over the past 4 decades. However, too many children still live in housing with deteriorated lead-based paint and are at risk for lead exposure with resulting lead-associated cognitive impairment and behavioral problems [AAP 2016; ACCLPP 2012].
The Exposure-Disease Model
No matter how toxic, no chemical can harm a person (child, adult, or both) unless exposure occurs. After a sufficient level of exposure (dose) to the chemical, biologic uptake, target organ contact, and biologic change can occur, all of which can lead to disease or other effects [ATSDR 2012; ATSDR 2013].
Steps that must occur for an environmental toxicant to cause disease.
- Environmental contamination (potential exposure): the exposure source and how the contaminant disperses in the environment.
- Exposure: For a toxicant to cause disease, exposure must occur. Exposure occurs through an exposure pathway between the contaminant in the physical environment and the exposed person.
- Biologic uptake: the process by which the transfer of substances from the environment to plants, animals, and humans occurs.
- Absorbed dose: how much of a toxicant is absorbed after an exposure occurs.
- Biologic changes: the chemical changes causing damage to tissues following a toxic exposure and an absorbed dose.
- Target organ: the organ or organs affected by an exposure to the toxicant. The “critical organ” is the most sensitive organ.
- Clinical disease: physical signs and symptoms resulting from a sufficiently absorbed toxicant dose.
Exposure Pathway
For a toxicant to cause disease, exposure must occur. Exposure occurs through an exposure pathway between the contaminant in the physical environment and the exposed person.
An exposure pathway has five parts.
- A source of contamination, such as an abandoned mine or industrial emissions.
- An environmental medium and transport mechanism, such as water or movement through a groundwater aquifer.
- A point of exposure, such as a private well.
- A route of exposure, such as
- eating,
- drinking,
- breathing,
- touching,
- transplacental exposure, and/or
- intravenous exposure.
- A receptor population, such as people potentially or actually exposed.
When all five parts are present, the exposure pathway is termed “a completed exposure pathway” [ATSDR 2005].
Lead serves no useful purpose in the human body. Its presence in the body can lead to toxic effects, regardless of age, gender, or exposure pathway.
The nervous system is the most sensitive organ system for lead exposure in children.
- Lead toxicity can affect every organ system.
- On a molecular level, proposed mechanisms for toxicity involve fundamental biochemical processes. These include lead’s ability to inhibit or mimic the actions of calcium (which can affect calcium-dependent or related processes) and to interact with proteins (including those with sulfhydryl, amine, phosphate, and carboxyl groups) [ATSDR 2010].
- Lead’s high affinity for sulfhydryl groups makes it particularly toxic to multiple enzyme systems including heme biosynthesis.
- The National Toxicology Program [NTP 2012], and the American Academy of Pediatrics [AAP 2016] have concluded that there is sufficient evidence for adverse health effects in children and adults at blood lead levels (BLLs) <5 micrograms per deciliter (µg/dL).
- “There is no identified threshold or safe level of lead in blood” [AAP 2016].
- It is important to control or eliminate all sources of lead in children’s environments to prevent exposure.
The sections below describe specific health effects for population groups and major organ systems and functions. Some of the information in the population group is repeated in the organ system specific sections.
From 2007 to 2010, approximately 2.6% of preschool children in the United States had a blood lead concentration 5 μg/dL (≥ 50 ppb), which represents about 535,000 U.S. children 1 to 5 years of age [AAP 2016].
- In children, there is no identified threshold or “safe” blood lead level below which no risk of poor developmental or intellectual function is expected.
Data from the NTP [2012] showed that the effect of concurrent BLLs on IQ may be greater than currently believed.
Lead inhibits the bodies of growing children from absorbing iron, zinc and calcium, minerals essential to proper brain and nerve development.
- Children often show no signs of lead toxicity until they are in school, even as late as middle school, as expectations for academic achievement increase.
- The practicing health care provider can distinguish overt clinical symptoms and health effects that come with high exposure levels on an individual basis.
- However, lack of overt symptoms does not mean “no adverse impact.”
- Lower levels of exposure have been shown to have many subtle adverse health effects.
- Medical research has established a connection between early childhood lead exposure and future criminal activity, especially of a violent nature [Wright et al. 2008; Needleman et al. 2002; Needleman et al. 1996]. Numerous studies link elevated bone or blood lead levels with aggression, destructive and delinquent behavior, attention deficit hyperactivity disorder and criminal behavior [Wright et al. 2008; Braun et al. 2006; Needleman et al. 2004; Needleman et al. 2002; Nevin 2000; Bellinger et al. 1994].
- Acute exposure to very high levels of lead may produce encephalopathy in children.
While the immediate health effect of concern in children is typically neurological, it is important to remember that childhood lead poisoning can lead to health effects later in life, including
- ADHD, delayed learning, and lower IQ (which will impact school performance),
- Developmental problems with their offspring,
- Hypertension,
- Renal effects, and
- Reproductive problems.
Adverse health effects occur in children at BLLs <5 μg/dL [AAP 2016; NTP 2012]. The most common include
- Attention-related behavioral problems,
- Decreased cognitive performance, and
- Greater incidence of problem behaviors.
In pregnant women, there is sufficient evidence that maternal BLLs <5 μg/dL are associated with reduced fetal growth or lower birth weight. BLLs <10 μg/dL are associated with decreased postnatal growth, and concurrent BLLs <10 μg/dL in children are associated with
- Reduced head circumference,
- Height, or
- Other indicators of growth and delayed puberty [NTP 2012].
There is inadequate evidence for an association between BLLs <10 μg/dL and cardiovascular effects in children of any age, or renal function in children <12 years of age [NTP 2012]. BLLs in this range may not predict poor outcomes for individual children, but are important predictors at the population level.
It must be emphasized that there is no known blood lead level for children without some level of risk for some of the adverse neurological effects of lead in children.
The neurological effects in an adult exposed to lead as an adult can be neuropathy, and may be different from those of an adult exposed to lead as a child when the brain was developing (more information for adults in following sections).
- Childhood neurological effects, including attention deficit hyperactivity disorder (ADHD), may persist into adulthood.
- Lead-exposed adults may also experience many of the neurological symptoms experienced by children, but shown at higher blood lead levels.
Other effects are reduced sperm count and hypertension [NTP 2012]. However, sufficient evidence shows that BLLs <5 μg/dL are associated with decreased renal function and that BLLs <10 μg/dL are associated with increased blood pressure and hypertension [NTP 2012].
Sufficient evidence shows that maternal BLLs <5 μg/dL are associated with reduced fetal growth or lower birth weight [NTP 2012].
Pregnant women who were lead-exposed as adults may also experience many of the neurological symptoms experienced by children, although at higher blood lead levels.
Some women may develop eclampsia and pre-eclampsia with pregnancy. Maternal blood lead levels may be important predictors of the risk of developing eclampsia and pre-eclampsia [NTP 2012]. There is sufficient evidence showing that BLLs <5 μg/dL are associated with decreased renal function and that BLLs <10 μg/dL are associated with increased blood pressure and hypertension [NTP 2012].
Lead exposure has been linked with various types of brain damage. These include
- Problems with thinking (cognition);
- Difficulties with organizing actions, decisions, and behaviors (executive functions);
- Abnormal social behavior (including aggression); and
- Difficulties in coordinating fine movements, such as picking up small objects (fine motor control) [Cecil et al. 2008].
Sufficient evidence in children shows that BLLs <5 µg/dL are associated with increased diagnosis of attention-related behavioral problems, greater incidence of problem behaviors, and decreased cognitive performance. This is indicated by
- Decreased IQ,
- Lower academic achievement, and
- Reductions in specific cognitive measures [NTP 2012].
Lead causes activation of protein kinase C (PKC) and binds to PKC more avidly than calcium (its physiologic activator). This creates problems with neurotransmitter release. Alteration of PKC function also affects second-messenger systems within the cell which may lead to future changes in gene expression and protein synthesis.
A large body of evidence associates a reduction in IQ performance and other neuropsychological defects with BLLs <10 µg/dL, including reduced hearing [Landrigan and Etzel 2014; NTP 2012; ATSDR 2010; Lanphear et al. 2005].
- Neurological adverse effects of lead in children have been documented at exposure levels once thought to cause no harmful effects, including those <10 µg/dL [Canfield 2003; CDC 1997a] and <5 µg/dL [NTP 2012; Lanphear et al. 2005].
- Low doses of lead can cause a broad range of functional problems such as loss of self-control, shortened attention span and a host of learning disorders that often cause lead-exposed children to perform poorly in school and ultimately to drop out [Bellinger 2008a; Bellinger 2008b; Chen et al. 2007; Wilson et al. 2006; Lanphear et al. 2005].
- Because otherwise asymptomatic individuals may experience neurological effects from lead exposure, clinicians should have a high index of suspicion for lead exposure, especially in the case of children with developmental delays.
Children suffer neurological effects from lead at much lower blood lead levels than adults.
- Subclinical neurological effects may occur at lower BLLs – at or below the regulatory standard of 10 µg/dL, in some cases – and it may not be possible to detect them on clinical examination at the time of the exposure or peak BLLs. Children often show no signs of lead toxicity until they are in school, even as late as middle school, when expectations for academic achievement increase.
- Some studies have found, for example, that for every 10 µg/dL increase in BLLs, children’s IQ was found to be lowered by 4 to 7 points [Winneke et al. 1990 as cited in AAP 1993; Fulton et al. 1987; Landsdown et al. 1986; Hawk et al. 1986; Schroeder et al. 1985; Yule et al. 1981].
- Evidence shows that ADHD and hearing impairment in children increase with increasing BLLs, and that lead exposure may disrupt balance and impair peripheral nerve function [ATSDR 2010].
In children, acute exposures leading to very high blood lead levels (< 70 µg/dL), may produce encephalopathy and other accompanying signs of
- Ataxia,
- Coma,
- Convulsions,
- Death,
- Hyperirritability, and
- Stupor.
The BLLs associated with encephalopathy in children vary from study to study, but BLLs of 70-80 µg/dL or greater appear to indicate a serious risk. Even without encephalopathy symptoms, these levels are associated with increased incidences of lasting neurological and behavioral damage [ATSDR 2010].
In adults, lead encephalopathy may occur at extremely high BLLs, e.g., 460 µg/dL [Kehoe 1961 as cited in ATSDR 2010].
- Precursors of encephalopathy may occur at lower BLLs, such as
- Dullness,
- Irritability,
- Loss of memory,
- Muscular tremor, and
- Poor attention span.
Less severe neurological and behavioral effects have been documented in lead-exposed workers with BLLs ranging from 40 to 120 µg/dL [ATSDR 2010]. These effects include
- Decreased libido,
- Depression/mood changes,
- Diminished cognitive performance,
- Diminished hand dexterity,
- Diminished reaction time,
- Diminished visual motor performance,
- Dizziness,
- Fatigue,
- Forgetfulness,
- Headache,
- Impaired concentration,
- Impotence,
- Increased nervousness,
- Irritability,
- Lethargy,
- Malaise,
- Paresthesia,
- Reduced IQ scores, and
- Weakness.
Late signs of lead intoxication such as slowed nerve conduction and forearm extensor weakness (wrist drop) are more typical signs in workers chronically exposed to high lead levels.
Childhood lead exposure is associated with region-specific reductions in adult gray matter volume. Affected regions include the portions of the prefrontal cortex and anterior cingulate cortex (ACC) responsible for executive functions, mood regulation, and decision-making [Cecil et al. 2008]. These neuroanatomical findings were more pronounced for males, suggesting that lead-related atrophic changes have a disparate impact across sexes. This analysis suggests that adverse cognitive and behavioral outcomes may be related to lead’s effect on brain development producing persistent alterations in structure. Using a simple model, Cecil et al. [2008] found that blood lead concentration mediates brain volume and fine motor function. As shown in Figure 3 (model uses composite data to represent areas of significant volume loss overlaid with mean childhood blood lead concentrations), lead exposure appears to have a larger effect on brain volume loss in men than in women, which might help to explain the higher incidence of antisocial behaviors among men than women. Overall, these findings may explain why children and adults who have a history of lead exposure have behavioral and other problems, and support ongoing efforts to reduce childhood lead exposure in the United States and other countries. More research is needed to support and expand upon these findings.
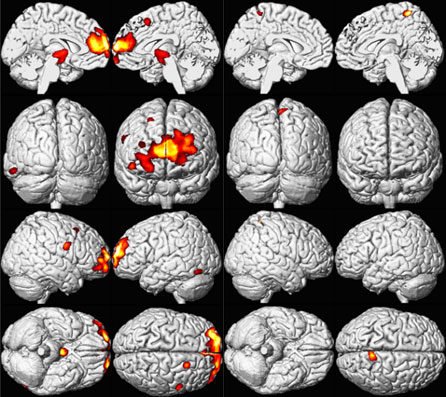
Figure 3. Sex Influences Brain Volume Loss Associated with Lead Exposure (Males left and Females right) [Cecil et al 2008].
A composite representation of regions with significant volume loss for Cincinnati Lead Study (CLS) participants associated with mean childhood blood lead concentrations is shown in red and yellow overlaid upon a standard brain template for males (n=83) and females (n=74). The model is adjusted for age at time of scanning and birth weight, using a cluster threshold of 700 voxels and unadjusted; ≤0.001 [Cecil et al 2008].
The lowest blood lead level at which lead has an adverse effect on the kidney remains unknown.
Many studies show a strong association between lead exposure and renal effects [NTP 2012; ATSDR 2010]. Latent effects of childhood lead exposure include chronic advanced renal disease or a reduction in renal function in adulthood.
Impaired renal function and kidney disease are reported at high levels of lead exposure, as estimated mainly through concentrations of serum creatinine (SCr) and rates of creatinine clearance from the body. However, a change in the serum creatinine is not sensitive for an early diagnosis of acute kidney injury [Han et al. 2008].
Urinary levels of matrix metalloproteinase-9 (MMP-9), N-acetyl-β-D-glucosaminidase (NAG), and kidney injury molecule-1 (KIM-1) have been used as biomarkers for the detection of acute kidney injury [Han et al. 2008].
- “Combining all three biomarkers achieved a perfect score diagnosing acute kidney injury” [Han et al 2008].
- Urinary biomarkers allow diagnosis of acute kidney injury earlier than a rise in serum creatinine.
BLLs of 1.5 μg/dL due to chronic low-level lead exposure reduces kidney function in adolescents. Doubling the blood lead level was associated with significant reduction in the glomerular filtration rate. “Chronic, low-level lead exposure is an important risk factor for chronic renal failure” [Landrigan and Etzel 2014].
Some population-based studies show accelerated increases in serum creatinine or reductions in creatinine clearance at BLLs below 60 μg/dL [Tsaih et al. 2004; Kim et al. 1996; Hu et al. 1994; Staessen et al. 1992].
- There is sufficient evidence that blood BLLs ‹5 μg/dL are associated with adverse effects on kidney function in adults (increased risk of chronic kidney disease, and decreases in the estimated glomerular filtration rate and creatinine clearance, markers of kidney function) [NTP 2012].
- At ‹10 μg/dL there is increased blood pressure and increased risk of hypertension in adults [NTP 2012]. However, continued or repetitive exposures can cause toxic stress on the kidney that, if unrelieved, may develop into chronic and often irreversible lead nephropathy (e.g., chronic interstitial nephritis).
- Lead nephrotoxicity is characterized by
- Proximal tubular nephropathy,
- Glomerular sclerosis, and
- Interstitial fibrosis [Zalups and Diamond 2004; Goyer 1985; Loghman-Adham 1997].
- Most documented renal effects for occupational workers have been observed in acute high-dose exposures and high-to-moderate chronic exposures (BLL ›60 μg/dL).
Some patients show Fanconi-like syndrome [Landrigan and Etzel 2014] (Fanconi syndrome consists of multiple defects in renal proximal tubular reabsorption, causing glucosuria, phosphaturia, generalized aminoaciduria, and bicarbonate wasting).
This nephropathy is most commonly reported in children, and even though it is reversible, should be treated to prevent further kidney damage. Treatment of acute lead nephropathy in children appears to prevent the progression to chronic interstitial nephritis [Weeden et al. 1986].
It should be noted that lead-induced end-stage renal disease is a relatively rare occurrence in the U.S. population today.
- Renal disease can be asymptomatic until the late stages and may not be detected unless appropriate tests are performed.
- Because past or ongoing excessive lead exposure may also be a causative agent in kidney disease associated with essential hypertension [ATSDR 2010], primary care providers should closely follow the renal function of patients with hypertension and a history of lead exposure (See the “Hypertension Effects” section).
In patients with lead nephropathy, uric acid excretion is substantially lower than would be expected on the basis of their glomerular filtration rate (GFR). Studies have suggested enhanced reabsorption and reduced secretion of uric acid, explaining a high prevalence of gout [Kathuria et al. 2016]. In one study, more than 50% of patients suffering from lead nephropathy also suffered from gout [Bennett 1985 as cited in ATSDR 2010].
Lead inhibits the body’s ability to make hemoglobin by interfering with several enzymatic steps in the heme synthesis pathway.
- Specifically, lead decreases heme biosynthesis by inhibiting d-aminolevulinic acid dehydratase (ALAD) and ferrochelatase (FECH) activity.
- Ferrochelatase, which catalyzes the insertion of iron into protoporphyrin IX, is quite sensitive to lead.
- A decrease in the activity of this enzyme results in an increase of the substrate erythrocyte protoporphyrin (EP) in the red blood cells (also found in the form of ZPP-bound to zinc rather than to iron).
- Also associated with lead exposure is an increase in blood and plasma d-aminolevulinic acid (ALA) and free erythrocyte protoporphyrins (FEP) [EPA 1986, as cited in ATSDR 2010].
EPA estimated the threshold BLL for a decrease in hemoglobin to be 50 µg/dL for occupationally exposed adults and approximately 40 µg/dL for children, although other studies have indicated a lower threshold (e.g., 25 µg/dL) for children [EPA 1986 as cited in ATSDR 2010]. Lead induced anemia in adults is not a common occurrence.
Recent data indicate that the EP level, which has been used in the past to screen for lead toxicity, is not sufficiently sensitive at lower levels of blood lead and is therefore not as useful as a screening test as previously thought (see “Blood Lead Levels (BLLs)” in the “Clinical Assessment-Diagnostic Tests and Imaging” section for further discussion of EP testing.).
Lead can induce 2 types of anemia, often accompanied by basophilic stippling of the erythrocytes [ATSDR 2010].
- Acute high-level lead exposure has been associated with hemolytic anemia.
- Frank anemia is not an early manifestation of lead exposure, and is evident only when the BLL is significantly elevated for prolonged periods.
- In chronic lead exposure, lead induces anemia by both interfering with heme biosynthesis and by diminishing red blood cell survival.
- The anemia of lead intoxication is hypochromic and normo -or microcytic with associated reticulocytosis.
The heme synthesis pathway, on which lead has an effect, is involved in many other processes in the body, including
- Endocrine,
- Hepatic,
- Neural, and
- Renal.
The impact of biochemical and enzyme changes (and their possible sequelae) that may exist at lower blood lead levels remains unclear.
Studies of children with high lead exposure have found that a strong inverse correlation exists between BLLs and vitamin D levels.
- Lead impedes vitamin D conversion into its hormonal form, 1, 25-dihydroxyvitamin D, which is largely responsible for the maintenance of extra-and intra-cellular calcium homeostasis.
- Diminished 1, 25-dihydroxyvitamin D, in turn, may impair cell growth, maturation, and tooth and bone development.
- In general, these adverse effects seem to be restricted to children with chronically high BLLs (most striking in children with BLLs ›62 μg/dL) and chronic nutritional deficiency, especially with regard to calcium, phosphorous, and vitamin D [Koo et al. 1991 as cited in ATSDR 2010].
Lead appears to have a minimal, if any, effect on thyroid function.
In severe cases of lead poisoning, children or adults may present with severe cramping abdominal pain (colic-like pain), which may be mistaken for an acute abdomen or appendicitis. Lead colic is a symptom of chronic lead poisoning and is associated with obstinate constipation.
A few population studies have shown a possible connection between lead exposure and other cardiovascular disorders including
- Ischemic coronary heart disease,
- Cerebrovascular accidents, and
- Peripheral vascular disease [Vaziri and Gonick, 2008].
Several epidemiological and clinical studies have found a link between chronic lead exposure and elevated blood pressure [Vaziri and Gonick, 2008].
Hypertension is a complex condition with many different causes and risk factors, including family history, age, weight, diet, and exercise habits.
- Lead exposure is one factor of many that may contribute to the onset and development of hypertension.
- Although low to moderate lead level exposures (BLLs <30 μg/dL) show only a low degree of association with hypertension, higher exposures (primarily seen in occupational settings) increase the risk for hypertensive heart disease and cerebrovascular disease as latent effects.
- One study found that adults who experienced lead poisoning as children had a significantly higher risk of hypertension 50 years later (relative to control adults without childhood lead exposure) [Hu 1991 as cited in ATSDR 2010]. The association has been shown in population-based studies with BLLs below 10 μg/dL. Data supports an association between lead exposure and elevations in blood pressure [Schwartz 1995 as cited in ATSDR 2010; Hu et al. 1996; Korrick et al. 1999; Victery et al. 1988].
Reproductive effects examined in the literature include
- Sperm count,
- Fertility, and
- Pregnancy outcomes.
While several studies have implicated lead as contributing to reproductive and developmental effects, these effects have not been well-established at low exposure levels.
Male Reproductive Effects
Recent reproductive function studies in humans suggest that current occupational exposures may decrease sperm count totals and increase abnormal sperm frequencies [NTP 2012].
- In men, there is sufficient evidence that BLLs ≥ 15 µg/dL are associated with adverse effects on sperm or semen.
- It is unclear how long these effects may last in humans after lead exposure ceases.
Fertility
There is sufficient evidence that BLLs ‹10 µg/dL show adverse health effects on reproduction in adult women, and BLLs ≥ 20 µ/dL are associated with delayed conception time [NTP 2012].
Pregnancy Outcomes
Prenatal lead exposure has known influences on maternal health and infant birth and neurodevelopmental outcomes [CDC 2010].
Recent National Health and Nutrition Examination Survey (NHANES) estimates suggest that almost 1% of women of childbearing age (15-44 years) have BLLs greater than or equal to 5 µg/dL [CDC 2010]. As documented in the CDC guidelines for the identification and management of lead exposure in pregnant and lactating women (https://stacks.cdc.gov/view/cdc/11854/Print), there is evidence that maternal lead exposure during pregnancy can cause fetal lead exposure and can adversely affect both maternal and child health across a wide range of maternal exposure levels.
There is consistent evidence that the risk for spontaneous abortion is increased by maternal exposure to high levels of lead (e.g., occupational exposures) [Hertz-Picciotto 2000].
Borja-Aburto [1999] showed a statistically significant dose-response relationship between maternal BLLs and risk for spontaneous abortion at lower levels of exposure (0 to 30 µg/dL).
The previous lack of evidence for an association at these low-to-moderate BLLs may be due to methodologic deficiencies in these studies, such as small sample sizes, lack of control for confounding, problems in case ascertainment, and/or limitations in exposure assessment [Hertz-Piccioto 2000].
- The strongest evidence to date is a prospective study of pregnant women in Mexico City.
- This study addressed most of the deficiencies of the prior studies and demonstrated a statistically significant dose-response relationship between maternal BLLs (average 11.0 µg/dL) and risk for spontaneous abortion [Borja-Aburto et al. 1999].
There is also limited evidence that maternal BLLs ‹ 10 µg/dL are associated with preterm birth and spontaneous abortion [NTP 2012].
Developmental effects examined in the literature include pregnancy outcomes (e.g., premature births and low birth weights), congenital abnormalities, and post-birth effects on growth or neurological development.
- Increasing evidence indicates that lead, which readily crosses the placenta, adversely affects fetal viability as well as fetal and early childhood development.
- Prenatal exposure to low lead levels (e.g., maternal BLLs of 14 μg/dL) may increase the risk of reduced birth weight and premature birth [ATSDR 2010].
- Lead is an animal teratogen, but the available data are inadequate to establish the presence or absence of an association between maternal lead exposure and major congenital anomalies [CDC 2010].
In a retrospective study, a higher proportion of learning disabilities were found among school-aged children with biological parents who had lead poisoning as children 50 years previously [Hu 1991].
Lower Bone Mineral Density (BMD)
Lead has been linked to problems with the development and health of bones. At high levels, lead can result in slowed growth in children.
There is a growing body of scientific literature that supports a role of lead exposure in the development of osteoporosis.
Rat studies have found that lead exposure is associated with decreased bone mineral density (BMD). However, human studies are limited. Lead-exposed animals had decreased bone mass that resulted in bones that were more susceptible to fracture. Lead decreased osteoblastic cell numbers leading to a depression of bone formation. Accompanying this, lead exposure elevated sclerostin protein levels in the skeleton, and correspondingly reduced levels of β-catenin and Runx2 in stromal precursor cells. Lead also increased skeletal expression of peroxisome proliferator-activated receptor-γ (PPAR-γ). These results indicate a shift in mesenchymal differentiation wherein lead promoted enhanced adipogenesis and decreased osteoblastogenesis. Substantial differences in bone marrow composition were observed, highlighted by an increase in adipocytes [Beier et al. 2013].
Human studies have shown both positive associations between BMD and blood lead levels in children (i.e., the higher the BMD, the higher the blood lead level) [Campbell et al. 2004] and negative [associations] in adults [Campbell and Auinger 2007; Nash et al. 2004], but these results have not allowed inferences about the likely causal direction of this relation [Theppeang et al. 2008].
Children with high lead exposure (mean, 23.6 μg/dL BLL), had a significantly higher BMD than did children with low lead exposure (mean, 6.5 μg/dL BLL). This may reflect a true phenomenon because lead exposure has been reported to accelerate bony maturation by inhibiting the effects of parathyroid hormone-related peptide. Accelerated maturation of bone may ultimately result in a lower peak BMD being achieved in young adulthood, thus predisposing to osteoporosis in later life.
Future studies need to investigate this proposed model [Campbell et al. 2004].
“There could be potential effect modification by BMD on the relations between blood and bone lead levels, an analysis directly relevant to hypotheses regarding BMD and release of lead from bone. A growing literature suggests that genetic polymorphisms may modify the toxicokinetics of lead in bone and blood” (i.e., VDR, APOE) [Theppeang et al. 2008].
Analysis of NHANES III data showed a significant inverse association between lead exposure and BMD, but because of NHANES’s cross sectional design, caution should be taken in drawing conclusions from these findings alone.
Dental Health
Researchers are currently investigating the impacts of lead on dental health. One study found pre-and perinatal exposure to lead increased prevalence of caries in rat pups by almost 40% [Watson et al. 1997].
Cancer
EPA has classified elemental lead and inorganic lead compounds as Group 2B: probable human carcinogens, revised in 2011 [EPA 2013b]. This classification is based in part on animal studies, which have been criticized because the doses of lead administered were extremely high.
The NTP [2004] classified lead and lead compounds as “reasonably anticipated to be a carcinogen”.
- Adverse health effects may occur in children and adults at BLLs <5 μg/dL.
- In children, health effects generally occur at lower BLLs than in adults.
- There is no identified threshold or safe level of lead in blood.
- The nervous system is the most sensitive organ system for lead exposure in children.
- The developing nervous system of a child can be affected adversely at BLLs of ≤5 μg/dL. Subclinical effects may be present, but may not be detectable during clinical examination.
- There is a wide range of neurological effects associated with lead exposure, some of which may be irreversible.
- Lead exposure can lead to renal effects such as Fanconi-like syndromes, chronic nephropathy, and gout.
- Most lead-associated renal effects or diseases result from ongoing chronic exposure, present high acute exposure, or as latent effects from past chronic lead exposure.
- Lead inhibits several enzymes critical to the synthesis of heme, causing a decrease in blood hemoglobin.
- Lead interferes with a hormonal form of vitamin D which affects multiple processes in the body, including cell maturation and skeletal growth.
- Lead exposure may lead to increased risk for hypertension and its sequelae.
- Evidence suggests an association between lead exposure and certain reproductive and developmental outcomes.
- Maternal blood lead, from exogenous and endogenous sources, can cross the placenta and pose a risk to the fetus.
- Other potential health effects of lead are currently being studied.