What Is Carbon Tetrachloride?
Carbon tetrachloride (CCl4) is a manufactured chemical that does not occur naturally in the environment. Its chemical structure is shown below:
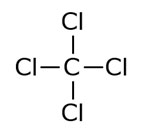
Figure 1. Carbon tetrachloride chemical structure
CCl4 is a clear, nonflammable, heavy liquid that evaporates readily, producing a sweet characteristic odor similar to chloroform.
CCl4 is classified as a volatile organic compound (VOC).
Common synonyms for carbon tetrachloride include
- Carbon tet,
- Freon 10,
- Perchloromethane,
- Tetrachloromethane. A more detailed list of synonyms is available from: https://chem.nlm.nih.gov/chemidplus/name/carbon%20tetrachloride%20%5Bnf%5D.
Historically, CCl4 was mainly used to produce chlorofluorocarbons (CFCs), which are used as heat transfer agents in refrigerating equipment and as aerosol propellants [Holbrook 1991]. In the United States, CCl4 was also an ingredient in many industrial fluids, and was an effective metal degreaser. It was found in household cleaning supplies and spot removers for carpets, clothing, and furniture [Doherty 2000; Odabasi 2008]. CCl4 was also used as an antihelminthic, a grain fumigant, and a component in fire extinguishers.
Carbon tetrachloride’s toxicity was recognized in the early 1900s [Abbott and Miller 1948; Hardin 1954], and most of the uses were discontinued by the mid-1960s. The U.S. Environmental Protection Agency cancelled CCl4‘s use as a fumigant in 1986. In the United States, CCl4 use is restricted to industrial and laboratory applications only [Seifert 1994; ATSDR 2005; NTP 2016]. It is not permitted in products intended for home use; however, chemicals containing CCl4 can still be purchased online [EPA 2017].
Both the internationally ratified Montreal Protocol (which first went into effect in 1989) and the United States 1990 Clean Air Act Amendments were instrumental in reducing environmental concentrations of CCl4 and other ozone-depleting chemicals [ATSDR 2005]. By 2009, the United States no longer regularly imported CCl4; only 90 pounds (41 kilograms) were imported during 1996-2009. Likewise, U.S. production and export of CCl4 dropped precipitously during this time [NTP 2016]. By 2009, only 26 manufacturers worldwide produced carbon tetrachloride, including three in the United States [NTP 2016].
- Most industrial CCl4 is used in the synthesis of CFCs and chlorinated solvents. Production of CCl4 has been continuously declining in response to regulation.
- Because of CCl4‘s toxicity, use in consumer products, and as a fumigant it is no longer permitted in the United States; only industrial uses and laboratory applications remain.