PFAS Information for Clinicians – 2024
- Communities around the United States have been concerned about possible health effects from PFAS exposure and have been looking to healthcare providers for counseling and support related to PFAS exposure.
- Ingestion of contaminated food and water is a main route of PFAS exposure.
- Health effects potentially associated with PFAS exposure include increases in cholesterol levels, decreases in birth weight, lower antibody response to vaccines, kidney and testicular cancer, pregnancy-induced hypertension, preeclampsia, and changes in liver enzymes.
- An exposure history can help clinicians determine the duration, magnitude, and routes of patients’ PFAS exposures and reveal opportunities for exposure reduction.
- In deciding whether to order PFAS testing, clinicians can consider
- an individual’s exposure history,
- results of PFAS testing from the patient’s water supply, food sources, or other exposure routes, and
- whether results can inform exposure reduction and health promotion.
- PFAS blood testing results do not provide information for treatment or predict future health problems.
- Patients and clinicians can discuss the potential risks and benefits of using PFAS blood testing results to guide clinical management. Considerations include
- factors unique to the patient, including the patient’s risk for disease,
- whether health screening beyond the usual standards of care is appropriate, and
- the potential for unnecessary further testing and treatment related to false positives from additional screening tests.
- No approved medical treatments are available to reduce PFAS in the body.
- ATSDR will continue to review the science and periodically update this information.
Per- and polyfluoroalkyl substances (PFAS) are a family of thousands of synthetic chemicals that all contain a partially or fully fluorinated carbon chain. Their chemical properties allow them to reduce friction and resist oil and water. As a result, they have been widely used in industry and consumer products since the 1940s. Major applications include surfactants used in industrial processes and firefighting foams, and protectants for paper packaging products, carpets, and textiles that enhance water, grease, and soil repellency.
PFAS are widespread and persistent in the environment. The carbon-fluorine bonds are strong, so these compounds do not fully break down in the environment or human body. In humans, properties of the most well-studied PFAS include the following:
- Absorption: Absorbed in the intestines and lungs; limited dermal absorption
- Distribution: Bind to serum proteins; to a lesser extent, also bind to tissue proteins (e.g., liver, kidneys, and brain)
- Metabolism: Most not metabolized; some metabolized to other PFAS
- Elimination: Mainly in urine (clearance rate can vary by sex and kidney function); also, through defecation, menstruation, breastfeeding, and placental transfer
- Half-life: A few days to 8 years or more, depending on the specific PFAS
Epidemiology
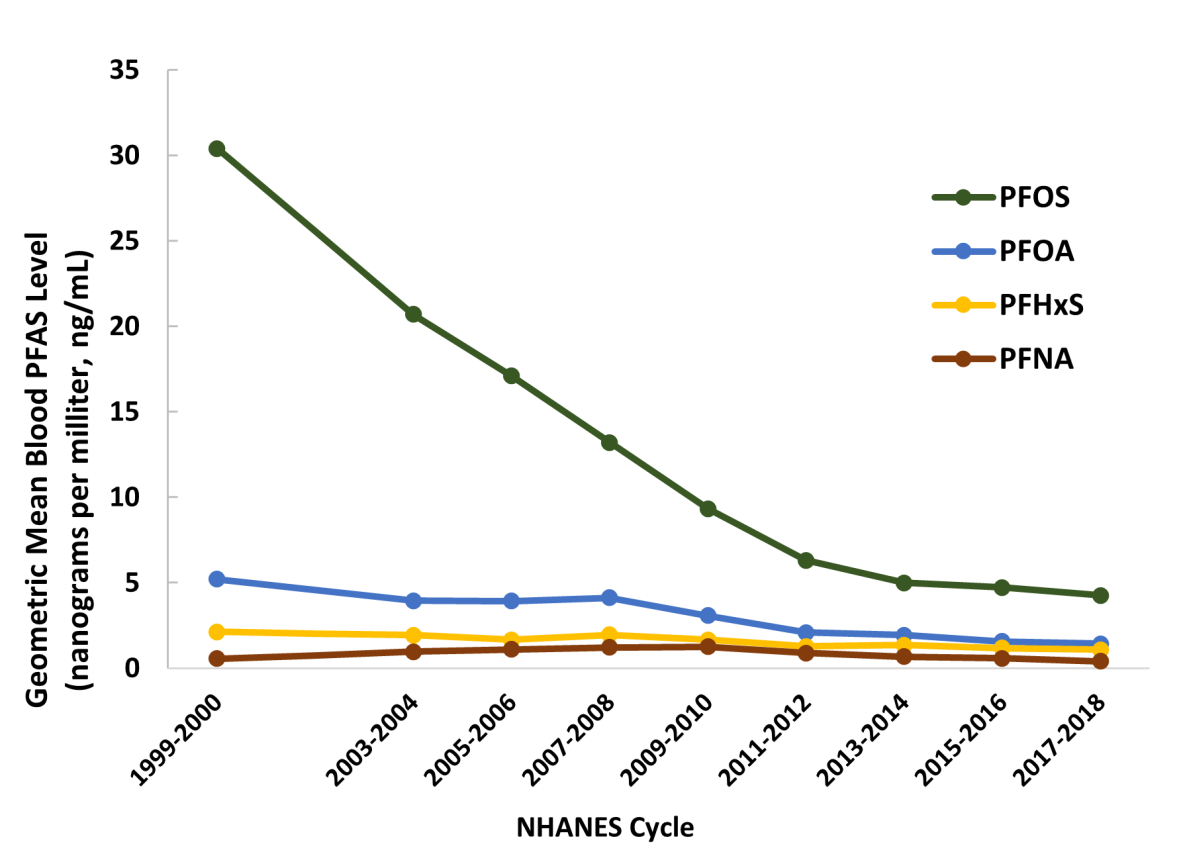
Nearly all people in the United States have measurable amounts of PFAS in their blood. Researchers often use people’s blood PFAS levels as a proxy for exposure. Since 1999, the National Health and Nutrition Examination Survey (NHANES) has been measuring certain PFAS (e.g., PFOS, PFOA, PFHxS, and PFNA) in blood samples from people living in the United States. The data show declining levels of three prevalent PFAS (PFOA, PFOS, and PFHxS), in part because the U.S. Environmental Protection Agency (EPA) enlisted major manufacturers to phase out production and reduce facility emissions of PFOA, PFOS, PFHxS, and PFNA. Population blood levels of substitute PFAS (e.g., GenX) are not well studied. Blood levels of these shorter-chain PFAS do not necessarily reflect total cumulative exposure because they tend to have relatively short half-lives.
In 2017–2018, NHANES reported geometric mean blood levels of
- PFOS: 4.25 ng/mL*, with 95% of the general population ≤14.6 ng/mL,
- PFOA: 1.42 ng/mL, with 95% of the general population ≤3.77 ng/mL,
- PFHxS: 1.08 ng/mL, with 95% of the general population ≤3.70 ng/mL, and
- PFNA: 0.411 ng/mL, with 95% of the general population ≤1.40 ng/mL.
*Nanograms per milliliter (ng/mL) is equivalent to micrograms per liter (µg/L) and to parts per billion (ppb).
Some communities can have higher geometric mean levels of blood PFAS than NHANES.
Exposure Sources and Routes
Communities with documented PFAS contamination in drinking water supplies or food are often near facilities that have manufactured, used, or handled PFAS; these include some factories, airports, military bases, wastewater treatment plants, farms where sewage sludge was used for fertilizer, landfills, or incinerators. Other PFAS exposure sources include PFAS-containing consumer products and workplaces that manufacture, use, or handle PFAS.
Routes of exposure to PFAS include ingestion, placental transfer, and inhalation. Dermal absorption of PFAS is limited and does not appear to be a significant route of exposure for the general population. For more information on occupational exposures, please visit the National Institute for Occupational Safety and Health (NIOSH) PFAS webpage.
Ingestion
Ingestion of food and water is a main route of PFAS exposure. In communities affected by PFAS-contaminated drinking water, water can be the main source of exposure. For other communities, the relative contribution of exposure sources can vary.
Ingestion of PFAS can occur through
- drinking water from PFAS-contaminated municipal sources or private wells,
- eating food (e.g., meat, dairy, and vegetables) produced near places where PFAS were used or made,
- eating fish caught from water contaminated by PFAS (PFOS, in particular),
- eating food from some types of grease-resistant paper or packaging (e.g., popcorn bags, fast food containers, pizza boxes, and candy wrappers), and
- swallowing contaminated soil.
Ingestion of residue and dust from PFAS-containing consumer products is another way people are exposed to PFAS. Research has suggested that exposure to PFOA and PFOS from today’s consumer products is generally lower than exposures from PFAS-contaminated drinking water. Some products that might contain PFAS include
- stain resistant carpets, upholstery, and other fabrics,
- water resistant clothing,
- cleaning products,
- personal care products and cosmetics (e.g., shampoo, dental floss, nail polish, and eye makeup), and
- paints, varnishes, and sealants.
Children can have higher PFAS exposures through ingestion than adults. Children eat and drink more relative to their body weight and can ingest dust or dirt containing PFAS through mouthing objects and hand to mouth behaviors. Children can also be exposed by
- drinking formula mixed with PFAS-contaminated water, and
- drinking breastmilk from persons exposed to PFAS.
Placental Transfer
Some PFAS cross the placenta and enter umbilical cord blood. The permeability of the placental barrier varies for different PFAS.
Inhalation
Most PFAS are not volatile, so inhalation is not a typical exposure route for the general population. People living near facilities that incinerate PFAS and PFAS-containing materials can be exposed to PFAS through inhalation.
In the workplace, handling of PFAS and PFAS-containing materials and breathing associated dust, aerosols, or fumes can also result in exposure to PFAS through inhalation. Inhalation is not a typical route of exposure for the general population but can occur with use of some PFAS-containing consumer products.
Drinking Water Standards, Regulations, and Health
Federal and state PFAS drinking water standards as well as health advisories for PFAS may differ and can be expected to change over time. In general, PFAS drinking water standards, regulations, and health advisories are not intended for use in assessing an individual patient’s health risks, but following these can help reduce drinking water exposures to PFAS.
Pathophysiology
The pathophysiology of PFAS toxicity is an area of active research. Many studies of populations have examined possible relationships between levels of PFAS in blood and rates of harmful health effects (see section below), but research has not yet confirmed mechanisms for each health effect. Complicating this challenge is that PFAS are sometimes examined as a class and other times examined as individual compounds.
Health Effects Associated with PFAS
The Agency for Toxic Substances and Disease Registry (ATSDR)’s 2021 Toxicological Profile for Perfluoroalkyls provides a comprehensive review of toxicological information for 12 different PFAS. ATSDR evaluated the available epidemiological data and found that the preponderance of the evidence suggested associations between exposure to individual PFAS and certain health effects.
Additionally, ATSDR and the National Institute of Environmental Health Sciences (NIEHS) funded the National Academies of Science, Engineering, and Medicine (The National Academies or NASEM) to provide an objective review of the current evidence regarding human health effects of PFAS. In its 2022 report Guidance on PFAS Testing and Health Outcomes, NASEM categorized the strength of evidence for various health effects for PFAS as a class. In addition to the health effects listed by ATSDR, NASEM also found epidemiologically-based associations with additional health effects.
Some animal and human studies find positive associations between PFAS exposure and a much wider range of health effects. For example, they identify associations with serum uric acid concentrations, reproductive health, diabetes, kidney effects, asthma, and neurobehavioral outcomes. Results of animal studies do not always correlate with human health effects because of physiologic differences between species. Inconsistent findings among human studies and study design limitations are some reasons why other potential health effects are not identified as associated with PFAS.
Health Effect
Health Effect
ATSDR Review of Associations (PFAS Associated with Health Effects)*
NASEM and ATSDR Health Effects
NASEM and ATSDR Health Effects
![]()
![]()
Increases in cholesterol levels
Increases in cholesterol levels
Evidence of an association (PFOA, PFOS, PFNA, PFDA)
Evidence of an association (PFOA, PFOS, PFNA, PFDA)
Sufficient evidence of an association
Sufficient evidence of an association
![]()
![]()
Small decreases in birth weight (<0.7-ounce decrease per 1 ng/mL blood PFOA/PFOS increase)
Small decreases in birth weight (<0.7-ounce decrease per 1 ng/mL blood PFOA/PFOS increase)
Evidence of an association (PFOA, PFOS)
Evidence of an association (PFOA, PFOS)
Sufficient evidence of an association
Sufficient evidence of an association
![]()
![]()
Lower antibody response to vaccines in children
Lower antibody response to vaccines in children
Evidence of an association (PFOA, PFOS, PFHxS, PFDA)
Evidence of an association (PFOA, PFOS, PFHxS, PFDA)
Sufficient evidence of an association
Sufficient evidence of an association
![]()
![]()
Kidney and testicular cancer
Kidney and testicular cancer
Evidence of an association (PFOA)
Evidence of an association (PFOA)
Sufficient evidence for kidney cancer / Limited or suggestive evidence for testicular cancer
Sufficient evidence for kidney cancer / Limited or suggestive evidence for testicular cancer
![]()
![]()
Pregnancy-induced hypertension or preeclampsia
Pregnancy-induced hypertension or preeclampsia
Evidence of an association (PFOA, PFOS)
Evidence of an association (PFOA, PFOS)
Limited or suggestive evidence of an association
Limited or suggestive evidence of an association
![]()
![]()
Changes in liver enzymes
Changes in liver enzymes
Evidence of an association (PFOA, PFOS, PFHxS)
Evidence of an association (PFOA, PFOS, PFHxS)
Limited or suggestive evidence of an association
Limited or suggestive evidence of an association
Additional Health Effects Considered
Additional Health Effects Considered
![]()
![]()
Thyroid disease and dysfunction
Thyroid disease and dysfunction
No consistent evidence of an association
No consistent evidence of an association
Limited or suggestive evidence of an association
Limited or suggestive evidence of an association
![]()
![]()
Breast cancer
Breast cancer
No consistent evidence of an association
No consistent evidence of an association
Limited or suggestive evidence of an association
Limited or suggestive evidence of an association
![]()
![]()
Ulcerative colitis
Ulcerative colitis
No consistent evidence of an association
No consistent evidence of an association
Limited or suggestive evidence of an association
Limited or suggestive evidence of an association
ATSDR and NASEM use different language to describe the strength of an association.
Many newer PFAS have properties, such as a shorter half-life, that limit the ability to use blood levels as a proxy for long-term exposure. This makes it more challenging to study whether exposure to these PFAS could cause health effects.
*ATSDR’s approach took into consideration the consistency of the findings across studies, the quality of the studies, dose-response, and plausibility. Although the data may provide evidence for an association, it does not always imply that the observed effect is biologically relevant because the magnitude of the change may be within the normal limits or not indicative of an adverse health effect. Causal relationships have not been established for these health effects.
†NASEM reviewed associations with PFAS as a class rather than by specific PFAS.
Factors Impacting the Risk of Health Effects
The risk of health effects associated with environmental exposures, including PFAS, depends on exposure factors (e.g., dose, frequency, route, and duration), individual factors (e.g., sensitivity to exposure and chronic disease burden), and other determinants of health (e.g., access to safer water and quality healthcare).
When patients have concerns about PFAS or other environmental exposures, clinicians can help address these concerns by actively listening and providing practical advice. Clinicians play an important role in helping patients identify and reduce exposures and in promoting standard age-appropriate preventive care measures for physical health, mental health, and wellness (e.g., Bright Futures and U.S. Preventive Services Task Force recommendations).
No approved medical treatments are available to remove PFAS from the body. Based on information from both a patient’s PFAS exposure history and the patient’s health history, clinicians can collaborate with patients to develop individualized care plans.
Clinical Presentation
PFAS toxicity is not associated with characteristic signs or symptoms. Patients with concerns about PFAS can present with a known exposure and be asymptomatic; they may have signs, symptoms, or a diagnosis of a disease or health issue (e.g., high cholesterol); or they may live in a community with exposure concerns but not know if they were exposed.
Exposure History
The goals of an exposure history are to
- Identify current and past PFAS exposures,
- Assess the duration, magnitude, and routes of exposure,
- Help patients understand how they have been exposed, and
- Determine if current exposures can be reduced.
A PFAS exposure history asks about the following:
- Documented PFAS contamination in the home, workplace, or community.
- Water, dietary, and consumer product exposure from
- contaminated drinking water (public water supplies or private well),
- fish from contaminated water,
- food wrapped or contained in grease-resistant paper or packaging, and
- PFAS-containing consumer products (see “Exposure Sources and Routes” section).
- Proximity to places that may manufacture, handle, or use PFAS, such as
- factories,
- airports,
- military bases,
- wastewater treatment plants,
- farms where sewage sludge was used for fertilizer,
- landfills, and
- incinerators.
- Occupational and recreational exposures to
- fluorochemical manufacturing processes,
- firefighting foams,
- ski wax, and
- other PFAS-containing materials.
- Past exposures.
- PFAS testing and results (e.g., drinking water or blood test results).
- Prenatal and infancy/childhood exposures including
- transplacental exposures, and
- breastmilk from a person exposed to PFAS or formula mixed with PFAS-contaminated water.
For any identified exposure, it is helpful to seek more detailed information about the route, dose, duration, and frequency of the exposure.
For more information, see ATSDR’s educational materials on Taking an Exposure History.
Exposure Reduction
An exposure history can inform how to reduce exposure, which is a central goal with any toxic exposure. At this time, it is not possible to eliminate all sources of PFAS exposure. With PFAS, the patient’s exposure might have come from contaminated drinking water or from other sources in their diet, home, or workplace. Local health departments may have information about area PFAS contamination concerns and can often provide additional resources and risk reduction strategies.
Exposure Source
Exposure Source
Example Exposure Reduction Strategies To Consider
Example Exposure Reduction Strategies To Consider
Drinking water
Drinking water
- Install a water filtration system or use a pitcher type filter shown to reduce PFAS levels.*
- Use an alternative water source tested for PFAS for drinking, food preparation (including infant formula), cooking, brushing teeth, or other activities that can result in ingestion of water.
- Test private well water. Consult local health or environmental agencies for guidance on how to get a private well tested, how to interpret results, and whether retesting is warranted. Consider installing a home water treatment system if needed.
- Install a water filtration system or use a pitcher type filter shown to reduce PFAS levels.*
- Use an alternative water source tested for PFAS for drinking, food preparation (including infant formula), cooking, brushing teeth, or other activities that can result in ingestion of water.
- Test private well water. Consult local health or environmental agencies for guidance on how to get a private well tested, how to interpret results, and whether retesting is warranted. Consider installing a home water treatment system if needed.
Fish, meat, eggs, or dairy
Fish, meat, eggs, or dairy
- Limit or avoid fish, meat, eggs, or dairy known to be contaminated with PFAS. Use local health advisories and EPA’s fish advisory list to guide choices.
- Limit or avoid fish, meat, eggs, or dairy known to be contaminated with PFAS. Use local health advisories and EPA’s fish advisory list to guide choices.
Food from contaminated fields or gardens
Food from contaminated fields or gardens
- Consume a wide variety of foods.
- For gardens, consider raised beds with alternate sources of soil and water.
- Consume a wide variety of foods.
- For gardens, consider raised beds with alternate sources of soil and water.
Dust in the home
Dust in the home
- Clean surfaces and floors frequently if soil around home is contaminated or if household members have occupational exposure to PFAS.
- Clean surfaces and floors frequently if soil around home is contaminated or if household members have occupational exposure to PFAS.
Consumer products
Consumer products
- When possible, choose products without PFAS (see “Exposure Routes and Sources” section).
- When possible, choose products without PFAS (see “Exposure Routes and Sources” section).
Workplace
Workplace
- Consult with an occupational and environmental medicine specialist.
- Consult with an occupational and environmental medicine specialist.
*If monitored, maintained, and used properly, a water filter can reduce PFAS levels. How much PFAS are removed by filtration is determined by the PFAS contaminant levels, the type of filter, and how well the filter is maintained. The global public health organization NSF International has developed a test method to verify a water filter’s ability to reduce PFOA and PFOS to below the health advisory levels set by the EPA or individual states. See NSF International’s list of approved devices.
For additional steps to reduce exposure, see EPA’s Meaningful and Achievable Steps You can Take to Reduce Your Risk.
PFAS Blood Testing
Systematic, community-wide blood testing can enable public health officials to investigate and respond to community-wide exposures. Results from these tests can assess the types and blood levels of PFAS in the community. (Blood PFAS is the accepted biomarker of exposure for PFAS studies, but some investigations have also included urine testing.)
Clinicians can order PFAS blood levels through CLIA-certified* commercial clinical laboratories. Results (current levels of PFAS in the blood) could reflect recent exposures or past exposures in the case of PFAS with long half-lives.
In deciding whether to order PFAS testing, clinicians can consider
- an individual’s exposure history,
- results of PFAS testing from the patient’s water supply, food sources, or other exposure routes, and
- whether results can inform exposure reduction and health promotion.
Benefits of PFAS blood testing might include
- information that could guide exposure reduction,
- greater recognition of PFAS-associated health effects, and
- possible psychological relief from knowing one’s PFAS blood level.
Limitations of PFAS blood testing include
- PFAS blood test results do not identify sources of exposure,
- only certain PFAS can be tested in blood and these PFAS might not represent the PFAS to which a patient has been exposed,
- results do not indicate whether a current illness can be attributed to PFAS exposure,
- PFAS blood test results do not predict future health outcomes,
- comparison of PFAS results across laboratories can be difficult (e.g., due to differences in assays used and PFAS tested),
- how long test results remain clinically meaningful is not known, and
- neither the utility of repeat PFAS testing nor the optimum interval for testing is known.
*For quality assurance, Clinical Laboratory Improvement Amendments (CLIA) regulate laboratory testing and require clinical laboratories to be certified by federal agencies before they can accept human samples.
ATSDR developed a PFAS Blood Level Estimation Tool for community members with exposure to PFAS through drinking water—in particular, for people who would like more information about how this exposure might affect blood PFAS levels. Estimates from this tool might be helpful when discussing potential PFAS exposures with patients.
Clinical Management Based on PFAS Blood Levels
Patients and clinicians can discuss the potential risks and benefits of using PFAS blood testing results to guide clinical management. Considerations include
- factors unique to the patient, including the patient’s risk for disease,
- whether health screening beyond the usual standards of care is appropriate, and
- the potential for unnecessary further testing and treatment related to false positives from additional screening tests.
ATSDR has not developed health-based screening based on PFAS blood levels and encourages clinicians to follow usual standards of care for health concerns.
For additional consideration, NASEM has proposed health screenings for patients exposed to PFAS based on the sum of certain PFAS (MeFOSAA, PFHxS, PFDA, PFUnDA, PFOS, PFOA, and PFNA) levels.† Providers who rely on NASEM thresholds for making decisions about further testing can consider that many people in the U.S. will exceed NASEM’s proposed thresholds for additional screening. Application of suggested cutoff levels based on PFAS blood testing could create changes in clinical care that differ from current preventive care guidelines.
†For additional consideration, NASEM has proposed using levels developed by the German Human Biomonitoring (HBM) Commission for PFOA and PFOS. The HBM Commission reviewed studies that did not include cancer as an outcome. NASEM incorporated the European Food Safety Authority (EFSA) methodology that used the sum of PFOS, PFOA, PFHxS, and PFNA to define a risk level and added other PFAS measured by NHANES into the summation. For patients with a sum of blood PFAS levels <2 ng/mL, NASEM recommends the usual standard of care. For blood PFAS levels 2 to <20 ng/mL, they encourage exposure reduction and screening for dyslipidemia, hypertension in pregnancy, and breast cancer. For blood PFAS levels ≥20 ng/mL, they suggest adding the following tests at all well visits: thyroid function testing with serum TSH for patients >18 years; an assessment for signs and symptoms of kidney cancer, including urinalysis, for patients >45 years; and an assessment for signs and symptoms of testicular cancer and ulcerative colitis in patients >15 years. NASEM estimated that for the U.S. population represented by NHANES in 2017–2018, 98% of people had PFAS levels ≥2 ng/mL; 9% had PFAS levels ≥20 ng/mL.
Pregnancy, Breastfeeding, and Children
Pregnancy – Exposure to PFAS can be associated with pregnancy-induced hypertension, preeclampsia, and small decreases in birth weight. These conditions can occur in many pregnancies, independent of PFAS exposure. Usual prenatal care, including monitoring a patient’s blood pressure closely, is appropriate.
Breastfeeding – PFAS can be found in human breast milk. Clinicians can assist patients in deciding whether to breastfeed based on factors specific to the patient and the child. Due to the many benefits of breastfeeding, the Centers for Disease Control and Prevention (CDC) and American Academy of Pediatrics recommend that most nursing people continue to breastfeed. More information on breastfeeding is available at Breastfeeding: Why It Matters.
Children – Studies have reported that exposure to certain PFAS is associated with a slightly lower immune response to some vaccines. The data do not suggest a need to reevaluate the usual immunization schedule (e.g., to repeat vaccinations).
For patients with PFAS exposure concerns, other professionals can help gather useful exposure information, evaluate the patients, recommend exposure reduction methods, and develop a patient monitoring or treatment plan. For example,
- Board-certified clinicians specializing in occupational and environmental medicine, medical toxicology, or pediatric environmental health can assist in evaluating and managing patients exposed or potentially exposed to hazardous substances.
- Occupational health clinicians (often at work sites) have knowledge about workplace exposures and health issues and can provide context or guidance to workers and clinicians.
- State or local health/environmental departments have knowledge about documented environmental contamination and associated health issues. They can help determine whether/how to assess health risks. They can also assist with identifying water quality reports and how to get private wells tested.
ATSDR will continue to review the science and periodically update PFAS information for clinicians.
ATSDR Toxicological Profile for PFAS
ATSDR PFAS Blood Level Estimation Tool
ATSDR Minimal Risk Levels for PFAS
CDC’s Breastfeeding: Why it Matters
CDC National Report on Human Exposure to Environmental Chemicals
EPA’s Meaningful and Achievable Steps You Can Take to Reduce Your Risk
NASEM Guidance on PFAS Testing and Health Outcomes
National Institute for Occupational Safety and Health PFAS webpage
American Academy of Pediatrics’ Bright Futures in Clinical Practice
AFFF
AFFF
Aqueous film forming foam
Aqueous film forming foam
ATSDR
ATSDR
Agency for Toxic Substances and Disease Registry
Agency for Toxic Substances and Disease Registry
CDC
CDC
Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
CLIA
CLIA
Clinical Laboratory Improvement Amendments
Clinical Laboratory Improvement Amendments
EFSA
EFSA
European Food Safety Authority
European Food Safety Authority
EPA
EPA
U.S. Environmental Protection Agency
U.S. Environmental Protection Agency
GenX
GenX
[Not an abbreviation; this is a trade name for a type of PFAS]
[Not an abbreviation; this is a trade name for a type of PFAS]
HBM
HBM
Human Biomonitoring
Human Biomonitoring
MeFOSAA
MeFOSAA
2-(N-Methyl-perfluorooctane sulfonamido) acetic acid
2-(N-Methyl-perfluorooctane sulfonamido) acetic acid
NASEM
NASEM
National Academy of Sciences, Engineering, and Medicine
National Academy of Sciences, Engineering, and Medicine
NHANES
NHANES
National Health and Nutrition Examination Survey
National Health and Nutrition Examination Survey
NIEHS
NIEHS
National Institute of Environmental Health Sciences
National Institute of Environmental Health Sciences
NIOSH
NIOSH
National Institute for Occupational Safety and Health
National Institute for Occupational Safety and Health
NSF
NSF
National Science Foundation
National Science Foundation
PFAS
PFAS
Per- and polyfluoroalkyl substances
Per- and polyfluoroalkyl substances
PFBS
PFBS
Perfluorobutane sulfonic acid
Perfluorobutane sulfonic acid
PFDA
PFDA
Perfluorodecanoic acid
Perfluorodecanoic acid
PFHxS
PFHxS
Perfluorohexane sulfonic acid
Perfluorohexane sulfonic acid
PFNA
PFNA
Perfluorononanoic acid
Perfluorononanoic acid
PFOA
PFOA
Perfluorooctanoic acid
Perfluorooctanoic acid
PFOS
PFOS
Perfluorooctane sulfonic acid
Perfluorooctane sulfonic acid
PFUnDA
PFUnDA
Perfluoroundecanoic acid
Perfluoroundecanoic acid
[ATSDR] Agency for Toxic Substances and Disease Registry. 2021. Toxicological profile for perfluoroalkyls. U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA [accessed 2023 May 4]. Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp200.pdf
[CDC] Centers for Disease Control and Prevention. 2023. National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention, Atlanta, GA [accessed 2023 May 4]. Available from: https://www.cdc.gov/exposurereport/index.html
[NASEM] National Academies of Sciences, Engineering, and Medicine. 2022. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington, DC: The National Academies Press. Available from: https://nap.nationalacademies.org/catalog/26156/guidance-on-pfas-exposure-testing-and-clinical-follow-up