Part 2: What are cholinesterase inhibitors?
- Learning Objectives
- The Primary Toxic Effect of Cholinesterase Inhibitors
- Breakdown of Acetylcholine by Cholinesterase (Optional Reading)
- Breakdown of Acetylcholine (Optional Reading)
- How Acetylcholine is Blocked (Optional Reading)
- Attraction of Cholinesterase Inhibitor to Acetylcholinesterase (Optional Reading)
- Molecular Bond Changes (Optional Reading)
- Cholinesterase Inhibitor Attached to Cholinesterase (Optional Reading)
- Effects of Blocked Acetylcholine Breakdown
- Other Effects of Cholinesterase Inhibitors (Optional Reading)
- Review of Definitions
- Two Classes of Cholinesterase Inhibitors
- Key Points
- Progress Check
Upon completion of this portion of the case study, you should be able to:
- Identify the chemical responsible for the acute pathology in cholinesterase inhibitor poisoning.
- Describe how cholinesterase inhibitors, including organophosphorus compounds (e.g., pesticides, nerve agents) and carbamates block the ability of acetylcholinesterase to break down acetylcholine.
Acetylcholinesterase inhibitors (which, for brevity, we will refer to as cholinesterase inhibitors) are chemicals whose primary toxic effect is to block the normal breakdown of the neurotransmitter, acetylcholine. This normal breakdown is shown in Figure 1 below.
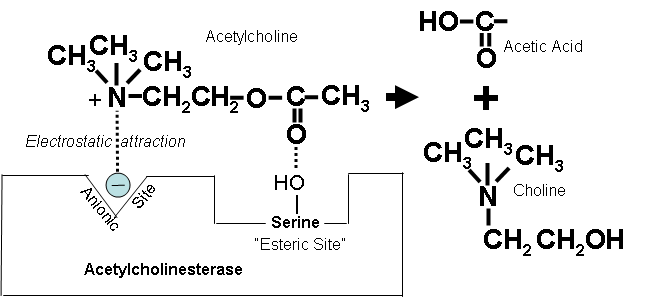
Figure 1. Breakdown of acetylcholine.
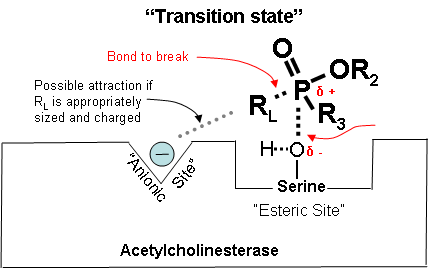
Figure 2 below shows how a cholinesterase inhibitor (in this case, a nerve agent) attaches to the serine hydroxyl group on acetylcholinesterase. This prevents acetylcholine from interacting with the cholinesterase enzyme and being broken down.
δ + Indicates that phosphorus is partially electropositive.
δ – Indicates that oxygen is partially electronegative.
Diagrams modified from Wiener, S. W., and R. S. Hoffman. “Nerve Agents: A Comprehensive Review.” Journal of Intensive Care Medicine 19, no. 1 (2004): 22-37.
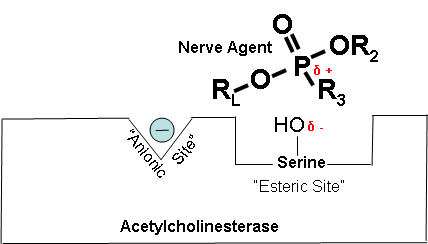
Figure 2. Partially electropositive phosphorus is attracted to partially electronegative serine.
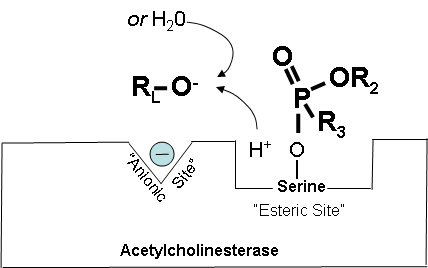
Figure 4. Cholinesterase inhibitor attached to acetylcholinesterase preventing the attachment of acetylcholine.
This leads to the build up of excessive levels of the neurotransmitter, acetylcholine, at the skeletal neuromuscular junction and those synapses where acetylcholine receptors are located
Thus, the primary manifestations of acute cholinesterase inhibitor toxicity are those of cholinergic (neurotransmitter) hyperactivity. (Carlton, Simpson et al. 1998)
There are also other delayed and chronic pathological effects of inhibitors of the cholinesterase enzyme which are less well understood.
Cholinesterase inhibitors can have effects on a variety of non-cholinesterase enzymes and neurotransmitters, as well. (Somani and Husain 2001) However, the significance of these effects is not well understood.
Acetylcholine: a chemical neurotransmitter found widely in the body. It triggers the stimulation of post-synaptic nerves, muscles, and exocrine glands.
Acetylcholinesterase (generally referred to as cholinesterase): an enzyme that rapidly breaks down the neurotransmitter, acetylcholine, so that it does not over-stimulate post-synaptic nerves, muscles, and exocrine glands.
Acetylcholinesterase inhibitor (generally referred to as cholinesterase inhibitor): a chemical that binds to the enzyme, cholinesterase, and prevents it from breaking down the neurotransmitter, acetylcholine. With toxic doses, the result is that excessive levels of the acetylcholine build up in the synapses and neuromuscular junctions and glands.
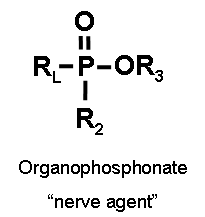
Two classes of cholinesterase inhibitors are organophosphorus compounds and carbamates. The key differences are listed in the table below. (Erdman 2004)
| Organophosphorus Compounds | Carbamates | |
|---|---|---|
| Molecular structure (Ecobichon 1996)
Notes: “R” denotes a variety of groups that attach to the basic structure. “P=S” of organophosphorus compounds can be substituted for “P=O.” “RL” of organophosphates may attach via an “O” to “P.” |
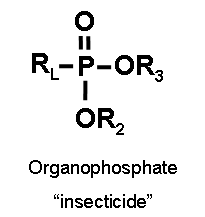
|
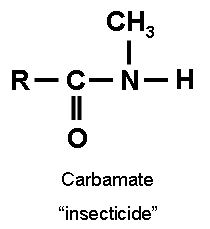 |
| Toxicity (Marrs and Dewhurst 2000) | Higher | Lower |
| Duration of action (Tareg, B et al. 2001) | Longer | Shorter |
| CNS toxicity (Tareg, B et al. 2001) | More common | Less common |
- Cholinesterase inhibitor toxicity is due to a decrease in the ability of cholinesterase to breakdown acetylcholine which results in excessively high acetylcholine levels.
- Cholinesterase inhibitors fall into two classes, organophosphorus compounds, and carbamates. The former are generally have higher toxicity, longer duration of action and more commonly cause CNS toxicity.